Dr Xiaoming Wang’s group revealed the non-canonical suppressive role of Jmjd1c in the plasma cell differentiation and rheumatoid arthritis
Source:Xiaoming Wang
2022-08-31
B cell differentiation into antibody-producing plasma cells is the essential process of humoral immunity and thus extensive studies have been done to understand the mechanism underlying B cell differentiation. The role of Blimp1, IRF4 and XBP1 as the drivers of plasma cell differentiation has now been well established. In contrast, the mechanism restraining plasma cell differentiation remain incompletely understood. Exaggerated B cell function and plasma cell generation is believed to play a key role in pathogenesis of autoimmune disorders, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
Jmjd1c, a member of JmjC-domain containing histone demethylase, was implicated in leukemia tumorigenesis, male gametogenesis and lipogenesis by removing H3K9me2 on target gene loci to promote gene transcription. Whether Jmjd1c plays a role in lymphocyte activation and differentiation has not been investigated. Genome-wide association analysis (GWAS) study suggested that the genetic variants around Jmjd1c gene locus is associated with RA and juvenile idiopathic arthritis (JIA), the most common chronic rheumatologic disease in children. However, the functional relevance and mechanistic basis of Jmjd1c in RA remain unexplored yet.
In Aug 2022, Dr Xiaoming Wang’s group at Nanjing Medical University, collaborated with Dr Nan Cha at the first affiliated hospital of Nanjing Medical University, published an article in Nature Immunology, entitled “Jmjd1c demethylates Stat3 to restrain plasma cell differentiation and rheumatoid arthritis”, revealed that Jmjd1c plays an essential role during B cell differentiation in a non-canonical way (Figure).
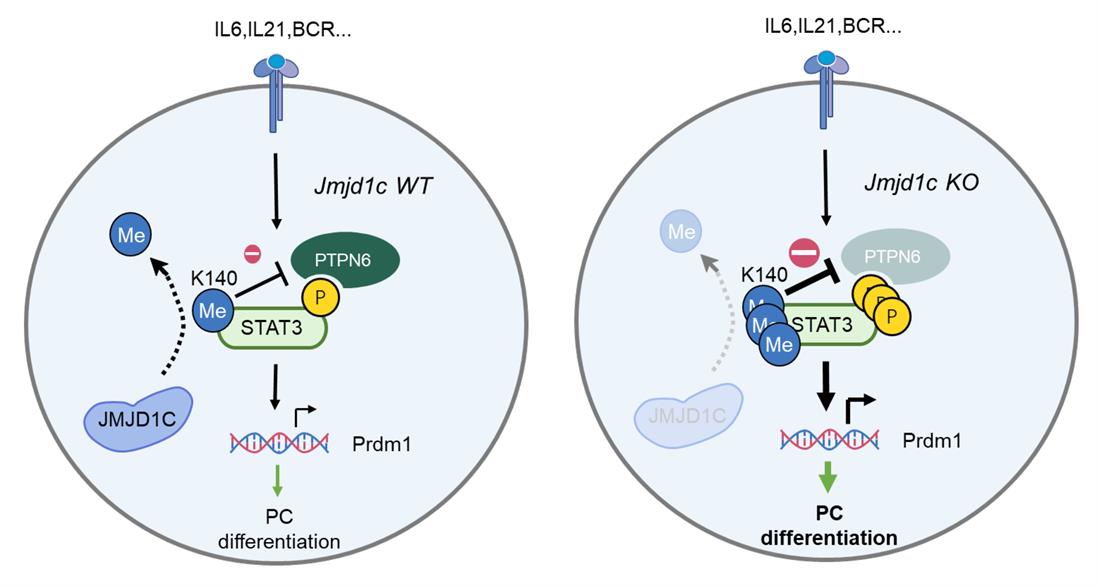
In this study, the investigators found that the expression of Jmjd1c, a member of JmjC-domain histone demethylase, in B cells but not in other immune cells, protected mice from rheumatoid arthritis. In human RA patients, Jmjd1c expression level in B cells was negatively associated with plasma cell frequency and disease severity. Mechanistically, Jmjd1c demethylated Stat3, rather than histone substrate, to restrain plasma cell differentiation. Stat3 K140 hypermethylation caused by Jmjd1c deletion inhibited the interaction with phosphatase Ptpn6 and resulted in abnormally sustained Stat3 phosphorylation and activity, which in turn promoted plasma cell generation. Germinal center B cells devoid of Jmjd1c also acquired strikingly increased propensity to differentiate into plasma cells. Stat3 K140R point mutation completely abrogated the effect caused by Jmjd1c loss. Mice with Jmjd1c overexpression in B cells exhibited opposite phenotypes to Jmjd1c deficient mice. Overall, this study revealed Jmjd1c as a critical regulator of plasma cell differentiation and rheumatoid arthritis and also highlighted the importance of demethylation modification for Stat3 in B cells. The study also JMJD1C might also serve as a biomarker for identifying patients with RA who are suited to B cell depletion therapy.
This study was supported by National Natural Science Foundation of China Grant, Jiangsu Outstanding Young Investigator Program and National Key R&D Program of China.
Links: https://www.nature.com/articles/s41590-022-01287-y
Jmjd1c, a member of JmjC-domain containing histone demethylase, was implicated in leukemia tumorigenesis, male gametogenesis and lipogenesis by removing H3K9me2 on target gene loci to promote gene transcription. Whether Jmjd1c plays a role in lymphocyte activation and differentiation has not been investigated. Genome-wide association analysis (GWAS) study suggested that the genetic variants around Jmjd1c gene locus is associated with RA and juvenile idiopathic arthritis (JIA), the most common chronic rheumatologic disease in children. However, the functional relevance and mechanistic basis of Jmjd1c in RA remain unexplored yet.
In Aug 2022, Dr Xiaoming Wang’s group at Nanjing Medical University, collaborated with Dr Nan Cha at the first affiliated hospital of Nanjing Medical University, published an article in Nature Immunology, entitled “Jmjd1c demethylates Stat3 to restrain plasma cell differentiation and rheumatoid arthritis”, revealed that Jmjd1c plays an essential role during B cell differentiation in a non-canonical way (Figure).

In this study, the investigators found that the expression of Jmjd1c, a member of JmjC-domain histone demethylase, in B cells but not in other immune cells, protected mice from rheumatoid arthritis. In human RA patients, Jmjd1c expression level in B cells was negatively associated with plasma cell frequency and disease severity. Mechanistically, Jmjd1c demethylated Stat3, rather than histone substrate, to restrain plasma cell differentiation. Stat3 K140 hypermethylation caused by Jmjd1c deletion inhibited the interaction with phosphatase Ptpn6 and resulted in abnormally sustained Stat3 phosphorylation and activity, which in turn promoted plasma cell generation. Germinal center B cells devoid of Jmjd1c also acquired strikingly increased propensity to differentiate into plasma cells. Stat3 K140R point mutation completely abrogated the effect caused by Jmjd1c loss. Mice with Jmjd1c overexpression in B cells exhibited opposite phenotypes to Jmjd1c deficient mice. Overall, this study revealed Jmjd1c as a critical regulator of plasma cell differentiation and rheumatoid arthritis and also highlighted the importance of demethylation modification for Stat3 in B cells. The study also JMJD1C might also serve as a biomarker for identifying patients with RA who are suited to B cell depletion therapy.
This study was supported by National Natural Science Foundation of China Grant, Jiangsu Outstanding Young Investigator Program and National Key R&D Program of China.
Links: https://www.nature.com/articles/s41590-022-01287-y


