The achievements of new type of immune cell differentiation were published by Chinese Scholars in “Immunity”
Source:Guangwei Liu
2016-07-04
On June 21, 2016, Professor Guangwei Liu from Institute of Cell Biology, College of Life Sciences, Beijing Normal University published their research team’s findings “histone deacetylase SIRT1 negatively regulates the differentiation of interleukin-9-producing CD4+ T cells” (Immunity, 2016, 44: 1337-1349) in the international first-class academic journal “Immunity”. This study revealed the regulatory effects and mechanisms of SIRT1 in Th9 cell differentiation, and laid the experimental and technical foundation for the study of pathogenesis and targeted therapy of Th9 cell associated asthma and tumor diseases.
Biological energy metabolism changes can regulate CD4+ T cell differentiation into different subsets and play a regulatory role in the clinical immune related diseases. This important immunological phenomenon has attracted the interest of scientists. Previous research has confirmed that Th1, Th2 and Th17 cells rely mainly on the regulation of glycolytic metabolism, and regulatory T (regulatory T; Treg) cell differentiation depends on regulation of lipid metabolism. However, the regulatory effects and mechanisms of Th9 cell differentiation remain unclear.
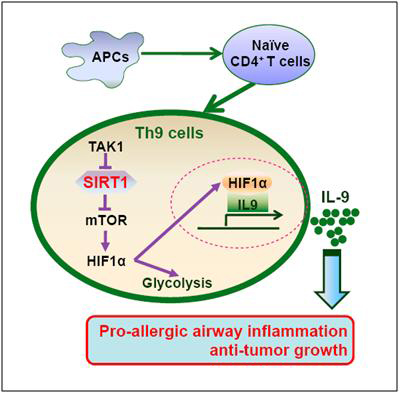
Chinese immunological researchers performed in-depth analysis about the roles and mechanisms of Th9 cells in allergic airway inflammation and tumor. They found that histone deacetylase SIRT1 negatively regulated the differentiation of Th9 cells and played a critical role in anti-tumor immunity and allergic airway inflammation. Glycolytic activation through the mTOR-hypoxia inducible factor-1α (HIF1α) is required for the differentiation of Th9 cells that conferred protection against tumors and is involved in allergic airway inflammation (Figure). This study defines the essential features of SIRT1-mTOR-HIF1α signaling-coupled glycolytic pathway in the induction of differentiation of Th9 cells, with implications for metabolic reprogramming as an immunotherapeutic approach.
This study was supported by the National Natural Science Foundation of China (81273201 and 31171407).
Article download address: http://www.cell.com/immunity/fulltext/S1074-7613(16)30163-7
Biological energy metabolism changes can regulate CD4+ T cell differentiation into different subsets and play a regulatory role in the clinical immune related diseases. This important immunological phenomenon has attracted the interest of scientists. Previous research has confirmed that Th1, Th2 and Th17 cells rely mainly on the regulation of glycolytic metabolism, and regulatory T (regulatory T; Treg) cell differentiation depends on regulation of lipid metabolism. However, the regulatory effects and mechanisms of Th9 cell differentiation remain unclear.
Chinese immunological researchers performed in-depth analysis about the roles and mechanisms of Th9 cells in allergic airway inflammation and tumor. They found that histone deacetylase SIRT1 negatively regulated the differentiation of Th9 cells and played a critical role in anti-tumor immunity and allergic airway inflammation. Glycolytic activation through the mTOR-hypoxia inducible factor-1α (HIF1α) is required for the differentiation of Th9 cells that conferred protection against tumors and is involved in allergic airway inflammation (Figure). This study defines the essential features of SIRT1-mTOR-HIF1α signaling-coupled glycolytic pathway in the induction of differentiation of Th9 cells, with implications for metabolic reprogramming as an immunotherapeutic approach.
This study was supported by the National Natural Science Foundation of China (81273201 and 31171407).
Article download address: http://www.cell.com/immunity/fulltext/S1074-7613(16)30163-7



