Prof. Chen Dong’s group reported the regulatory mechanism of miRNA to pathogenic T helper 17 (Th17) cells
Source:Chen Dong
2016-07-08
The group of Dr. Chen Dong at Tsinghua University Institute for Immunology identified microRNA mir-183Cas a novel regulator of pathogenic Th17 cell differentiation and function in autoimmune diseases. This finding is published in June 2016 issue of Immunity entitled “The MicroRNA-183-96-182 Cluster Promotes T Helper 17 Cell Pathogenicity by Negatively Regulating Transcription Factor Foxo1 Expression”. The work was led by a former postdoctoral fellow of Dr. Dong, Dr. Kenji Ichiyama, who is currently Assistant Professor in Osaka University in Japan. Dr. Chen Dong and Dr. Changchun Xiao from The Scripps Research Institute are the corresponding authors of this paper.
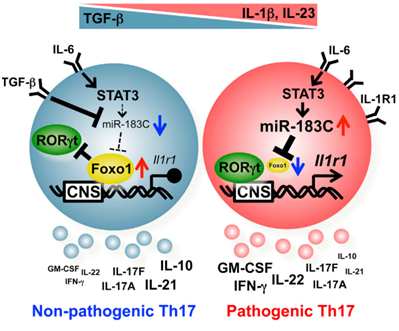
Th17 cell is a new T helper cell subset that plays important role in both immunity and inflammation. Under the control of master transcription factor RORgt, Th17 cells express signature cytokines IL-17A, IL-17F and GM-CSF to combat pathogens especially fungus while also drives inflammation in many autoimmune settings such as experimental autoimmune encephalomyelitis (EAE). It is recently discovered that IL-6, IL-23 and IL-1 signaling can together induce pathogenic Th17 cells contributing to autoimmunity, whereas the well known IL-6 plus TGF-b signals promote non-pathogenic Th17 cell differentiation. Understanding the regulatory mechanisms of Th17 pathogenesis will be of great value to provide novel therapeutic strategies towards treating autoimmune diseases without sacrificing normal immune function.
In this study, Dr. Dong's group found that deletion of the endoribonuclease-encoding Dicer1 specifically in Th17 cells protected mice from EAE, suggesting microRNAs are important regulators of Th17 pathogenesis. They further demonstrated that the Dicer1-regulated microRNA (miR)-183-96-182 cluster (miR-183C) can be induced by IL-6-STAT3 signaling and is highly expressed selectively in pathogenic Th17 cells but not in non-pathogenic Th17 cells such as those in steady state intestinal lamina propria. miR-183C expression enhanced pathogenic cytokine production from Th17 cells during their development and promoted autoimmunity. Mechanistically, miR-183C directly repressed expression of the transcription factor Foxo1, which negatively regulates the Th17 pathogenicity in part by inhibiting the expression of cytokine receptor IL-1R1. These findings reveal a novel mechanism of pathogenic Th17 differentiation and function regulated by microRNAs.
The paper links:http://www.cell.com/immunity/fulltext/S1074-7613(16)30203-5
Dr. Dong is the Director of Tsinghua University Institute for Immunology, Professor and Executive Dean of Tsinghua University School of Medicine. Dr. Dong’s lab is mainly focused on understanding the molecular mechanisms that immune and inflammatory responses are normally regulated, and applying this knowledge to the treatment of autoimmunity, allergic disorders and cancer. The work from Dr. Dong’s group has led to the discoveries of Th17 and T follicular helper (Tfh) cell subsets in the immune system and elucidation of their biological and pathological functions. He has published over 190 papers being cited by over 18000 times. He received the American Association of Immunologists BD Bioscience Investigator Award in 2009 and was elected as Fellow of American Association for the advancement of Science in 2011. In 2014 and 2015, Dr. Dong was awarded as Highly Cited Researcher by Thomson Reuters.
Th17 cell is a new T helper cell subset that plays important role in both immunity and inflammation. Under the control of master transcription factor RORgt, Th17 cells express signature cytokines IL-17A, IL-17F and GM-CSF to combat pathogens especially fungus while also drives inflammation in many autoimmune settings such as experimental autoimmune encephalomyelitis (EAE). It is recently discovered that IL-6, IL-23 and IL-1 signaling can together induce pathogenic Th17 cells contributing to autoimmunity, whereas the well known IL-6 plus TGF-b signals promote non-pathogenic Th17 cell differentiation. Understanding the regulatory mechanisms of Th17 pathogenesis will be of great value to provide novel therapeutic strategies towards treating autoimmune diseases without sacrificing normal immune function.
In this study, Dr. Dong's group found that deletion of the endoribonuclease-encoding Dicer1 specifically in Th17 cells protected mice from EAE, suggesting microRNAs are important regulators of Th17 pathogenesis. They further demonstrated that the Dicer1-regulated microRNA (miR)-183-96-182 cluster (miR-183C) can be induced by IL-6-STAT3 signaling and is highly expressed selectively in pathogenic Th17 cells but not in non-pathogenic Th17 cells such as those in steady state intestinal lamina propria. miR-183C expression enhanced pathogenic cytokine production from Th17 cells during their development and promoted autoimmunity. Mechanistically, miR-183C directly repressed expression of the transcription factor Foxo1, which negatively regulates the Th17 pathogenicity in part by inhibiting the expression of cytokine receptor IL-1R1. These findings reveal a novel mechanism of pathogenic Th17 differentiation and function regulated by microRNAs.

The paper links:http://www.cell.com/immunity/fulltext/S1074-7613(16)30203-5
Dr. Dong is the Director of Tsinghua University Institute for Immunology, Professor and Executive Dean of Tsinghua University School of Medicine. Dr. Dong’s lab is mainly focused on understanding the molecular mechanisms that immune and inflammatory responses are normally regulated, and applying this knowledge to the treatment of autoimmunity, allergic disorders and cancer. The work from Dr. Dong’s group has led to the discoveries of Th17 and T follicular helper (Tfh) cell subsets in the immune system and elucidation of their biological and pathological functions. He has published over 190 papers being cited by over 18000 times. He received the American Association of Immunologists BD Bioscience Investigator Award in 2009 and was elected as Fellow of American Association for the advancement of Science in 2011. In 2014 and 2015, Dr. Dong was awarded as Highly Cited Researcher by Thomson Reuters.


