Prof. Zhengfan Jiang’s group Discovered the Regulatory Mechanism to Keep Balance Between Type I-IFN and Inflammasome Signaling Pathways
Source:Zhengfan Jiang
2017-04-05
Recently, Prof. Zhengfan Jiang (School of Life Sciences, Peking University) has published a paper in Immunity about the novel discovery of the regulatory mechanism to keep balance between Type I-IFN and Inflammasome signaling pathways (Wang, Y. andNing, X., et al. (2017). Inflammasome Activation Triggers Caspase-1-Mediated Cleavage of cGAS to Regulate Responses to DNA Virus Infection. Immunity 46, 393-404.)
Viral infection triggers host innate immune responses that result in the production of various cytokines including type I interferons (IFN), activation of inflammasomes, and programmed cell death of the infected cells. For cytoplasmic DNA sensing, it has been discovered that cGAS recognizes cytosolic DNA and produces cGAMP, which functions as a second messenger to activate STING (MITA/ERIS), and triggers the activation of transcription factors NF-κB and IRF-3 or IRF-7 and the production of various cytokines including type I-IFNs. Microbial infection also causes inflammasome activation, which leads to the activation of caspase-1, and subsequent proteolytic conversion of proinflammatory cytokines, IL-1β and IL-18 from their precursors. Although type I-IFNs are critical for suppressing the spread of viral infection, aberrant production of these cytokines can have pathological roles in a variety of autoimmune disorders.Importantly, since microbial infection usually leads to type I-IFN production and inflammasome activation at the same time, balance between these key pathways is essential for immune homeostasis.
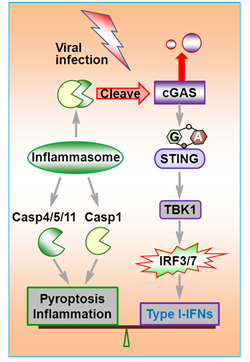
In this paper, Prof. Jiang’s group reported that caspases controlled antiviral immunity through cGAS cleavage during inflammasome activation. The authors found that inflammasome activation attenuates DNA virus-triggered type I-IFN signaling in both human and mouse cells. Consequently, caspase-1-, AIM2- and ASC-deficient mice showed enhanced resistance to infection by DNA but not RNA virus. Further investigation showed that upon inflammasome activation, caspase-1 directly bound to and cleaved human cGAS at D140/157, resulting in reduced cGAMP production and cytokine expression. Importantly, they also found that cGAS N-terminal R/K-rich region between residues 122 to 133 of human cGAS and residues 119 to 132 of mouse cGAS was critical for its normal function, which totally convers the previous understanding of cGAS N-terminal structure and function.
Previous work demonstrated that type I-IFN can inhibit inflammasome activation. This new finding reveals a role for inflammasomes in the dampening of IFN activating pathways and suggests a double-negative feedback loop that regulates the output of DNA-sensing pathways. Moreover, because numerous caspase inhibitors are under clinical trials, this paper suggested that these inhibitors’ potential effect as immune boosters should be closely investigated and efforts should be made to avoid unwanted innate immune activation.
Ph.D. student Yutao Wang and XiaohanNing from Peking University are the co-first authors of this paper. Prof. Zhengfan Jiang is the corresponding author. Prof. Xiaodong Su (School of Life Sciences, Peking University) and Researcher GuangxunMeng (Institut Pasteur of Shanghai, Chinese Academy of Sciences) participate in the study.This work was supported by grants from the Natural Science Foundation of China, National Program on Key Basic Research Project (973 Program) and Peking-Tsinghua Center for Life Sciences.
Full Text Link:http://www.cell.com/immunity/fulltext/S1074-7613(17)30073-0
Viral infection triggers host innate immune responses that result in the production of various cytokines including type I interferons (IFN), activation of inflammasomes, and programmed cell death of the infected cells. For cytoplasmic DNA sensing, it has been discovered that cGAS recognizes cytosolic DNA and produces cGAMP, which functions as a second messenger to activate STING (MITA/ERIS), and triggers the activation of transcription factors NF-κB and IRF-3 or IRF-7 and the production of various cytokines including type I-IFNs. Microbial infection also causes inflammasome activation, which leads to the activation of caspase-1, and subsequent proteolytic conversion of proinflammatory cytokines, IL-1β and IL-18 from their precursors. Although type I-IFNs are critical for suppressing the spread of viral infection, aberrant production of these cytokines can have pathological roles in a variety of autoimmune disorders.Importantly, since microbial infection usually leads to type I-IFN production and inflammasome activation at the same time, balance between these key pathways is essential for immune homeostasis.

In this paper, Prof. Jiang’s group reported that caspases controlled antiviral immunity through cGAS cleavage during inflammasome activation. The authors found that inflammasome activation attenuates DNA virus-triggered type I-IFN signaling in both human and mouse cells. Consequently, caspase-1-, AIM2- and ASC-deficient mice showed enhanced resistance to infection by DNA but not RNA virus. Further investigation showed that upon inflammasome activation, caspase-1 directly bound to and cleaved human cGAS at D140/157, resulting in reduced cGAMP production and cytokine expression. Importantly, they also found that cGAS N-terminal R/K-rich region between residues 122 to 133 of human cGAS and residues 119 to 132 of mouse cGAS was critical for its normal function, which totally convers the previous understanding of cGAS N-terminal structure and function.
Previous work demonstrated that type I-IFN can inhibit inflammasome activation. This new finding reveals a role for inflammasomes in the dampening of IFN activating pathways and suggests a double-negative feedback loop that regulates the output of DNA-sensing pathways. Moreover, because numerous caspase inhibitors are under clinical trials, this paper suggested that these inhibitors’ potential effect as immune boosters should be closely investigated and efforts should be made to avoid unwanted innate immune activation.
Ph.D. student Yutao Wang and XiaohanNing from Peking University are the co-first authors of this paper. Prof. Zhengfan Jiang is the corresponding author. Prof. Xiaodong Su (School of Life Sciences, Peking University) and Researcher GuangxunMeng (Institut Pasteur of Shanghai, Chinese Academy of Sciences) participate in the study.This work was supported by grants from the Natural Science Foundation of China, National Program on Key Basic Research Project (973 Program) and Peking-Tsinghua Center for Life Sciences.
Full Text Link:http://www.cell.com/immunity/fulltext/S1074-7613(17)30073-0


