Prof. Xing Chang’s Group Revealed New Pathogenic Mechanism of Autoimmune Disease
Source:Xing Chang
2018-07-28
On Jun 27th, Professor Xing Chang’s group from Shanghai Institute of Nutrition and Health,Chinese Academy of Sciences, published a paper in Immunity titled "Iron drives T helper cells pathogenicity by promoting RNA-binding protein PCBP1-mediated proinflammatory cytokine production." This paper reports that intracellular iron drives autoimmune disease by increasing proinflammatory cytokine production,and RNA-binding protein PCBP1 bridges iron metabolism and cytokine expression in T cells through post-transcriptional mechanisms.
Autoimmune diseases are disorders that the immune system mistakenly attacks and damages self-tissues. There are more than 100 types autoimmune disease,affecting approximately 5-8 percent of the people in the word. Unfortunately,there is little effective treatments to cure autoimmune diseases, because of poorly understood of those diseases. Iron deposition is frequently observed in human autoinflammatory diseases, such as in the central nervous system of patients with multiple sclerosis or in the blood of patients with rheumatoid arthritis. Yet, the functional outcome of excessive iron in the auto-inflammatory response is largely unknown.
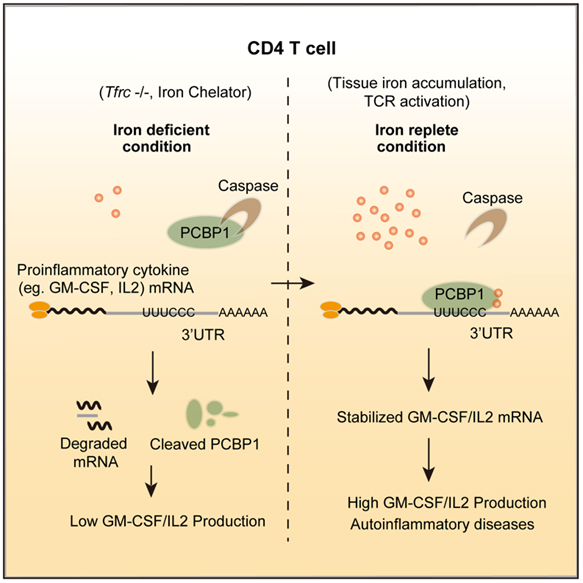
In this study, the researchers demonstrate that iron is able to directly participate in inflammatory responses by promoting proinflammatory cytokine production in the T cells via poly(rC)-binding protein 1 (Pcbp1), an RBP with iron chaperon activity. Intracellular iron depletion or Pcbp1 deficiency in autoreactive T cells resulted in shortened mRNA half-life and diminished GM-CSF production, protecting mice against EAE. Conversely, iron overload induced by iron dextran enhanced GM-CSF production of T cells via Pcbp1 in EAE. Mechanistically, intracellular iron protected Pcbp1 protein from caspase-mediated proteolysis, and Pcbp1 promoted mRNA stability by recognizing UC-rich elements in the 3’UTRs. These results suggest a model that excessive iron may directly precipitate autoimmune diseases through post-transcriptional regulation of pro-inflammatory cytokine expression in T cells.
Prof. Xing Chang’s group has been working on the mechanisms of lymphocytes development、differentiation and their impact on autoimmune disease for many years. They are also harnessing unique properties of lymphocytes to develop novel genetic methods to study post-transcriptional regulation and immune disorders. During the past few years, they had achieved a series of research findings,with the related results published in several high-profile journals, including Nature Methods,Proc Natl Acad Sci USA, eLife, Immunity.
Dr. Zhizhang Wang, a postdoctoral fellow, is the first authors of this paper. This work was supported by 2014CB943600 from MOST, 31370858 and 81671552 from NSFC, and 13PJ1409300 from SMSTC.
Autoimmune diseases are disorders that the immune system mistakenly attacks and damages self-tissues. There are more than 100 types autoimmune disease,affecting approximately 5-8 percent of the people in the word. Unfortunately,there is little effective treatments to cure autoimmune diseases, because of poorly understood of those diseases. Iron deposition is frequently observed in human autoinflammatory diseases, such as in the central nervous system of patients with multiple sclerosis or in the blood of patients with rheumatoid arthritis. Yet, the functional outcome of excessive iron in the auto-inflammatory response is largely unknown.

In this study, the researchers demonstrate that iron is able to directly participate in inflammatory responses by promoting proinflammatory cytokine production in the T cells via poly(rC)-binding protein 1 (Pcbp1), an RBP with iron chaperon activity. Intracellular iron depletion or Pcbp1 deficiency in autoreactive T cells resulted in shortened mRNA half-life and diminished GM-CSF production, protecting mice against EAE. Conversely, iron overload induced by iron dextran enhanced GM-CSF production of T cells via Pcbp1 in EAE. Mechanistically, intracellular iron protected Pcbp1 protein from caspase-mediated proteolysis, and Pcbp1 promoted mRNA stability by recognizing UC-rich elements in the 3’UTRs. These results suggest a model that excessive iron may directly precipitate autoimmune diseases through post-transcriptional regulation of pro-inflammatory cytokine expression in T cells.
Prof. Xing Chang’s group has been working on the mechanisms of lymphocytes development、differentiation and their impact on autoimmune disease for many years. They are also harnessing unique properties of lymphocytes to develop novel genetic methods to study post-transcriptional regulation and immune disorders. During the past few years, they had achieved a series of research findings,with the related results published in several high-profile journals, including Nature Methods,Proc Natl Acad Sci USA, eLife, Immunity.
Dr. Zhizhang Wang, a postdoctoral fellow, is the first authors of this paper. This work was supported by 2014CB943600 from MOST, 31370858 and 81671552 from NSFC, and 13PJ1409300 from SMSTC.


