Prof. Erwei Song and Shicheng Su’s group revealed a new mechanism of tumor immune evasion regulated by an lncRNA
Source:Erwei Song
2018-10-12
In October 2018, the research team directed by Professor Erwei Song and Shicheng Su from Sun Yat-sen Memorial Hospital published a paper in Nature Immunology titled ‘NKILA LncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death’. This paper reports that anti-tumor CTLs and TH1 cells, rather than pro-tumor TH2 and regulatory T cells, were sensitive to tumor-mediated apoptosis, which can be exploited by cancers to escape immunological destruction. Mechanistically, the lncRNA NKILA, regulated by IFN-STAT1 and TCR-calcium signaling, promotes T cell sensitivity to tumor-mediated apoptosis by inhibiting NF-κB activity. Moreover, engineering this lncRNA in adoptively transferred T cells might provide a novel antitumor immunotherapy.
Previous studies have demonstrated that immune surveillance is responsible for recognizing and eliminating the tumors, but tumors have acquired the ability to circumvent immune recognition and/or destruction through many different mechanisms. To overcome tumor immune escape, the most well known treatment is cancer immunotherapy, including immune checkpoint inhibitors and adoptive cell transfer therapy. Cancer immunotherapy was picked as the outstanding scientific achievement by Science’s top 10 breakthroughs of year 2013 and 2017, and won the 2018 Nobel Prize in Physiology or Medicine, which has been proven effective against hard-to-treat lymphoma and melanoma. However, immunotherapy is less effective in solid cancer, suggesting that the underlying mechanisms need further investigation.
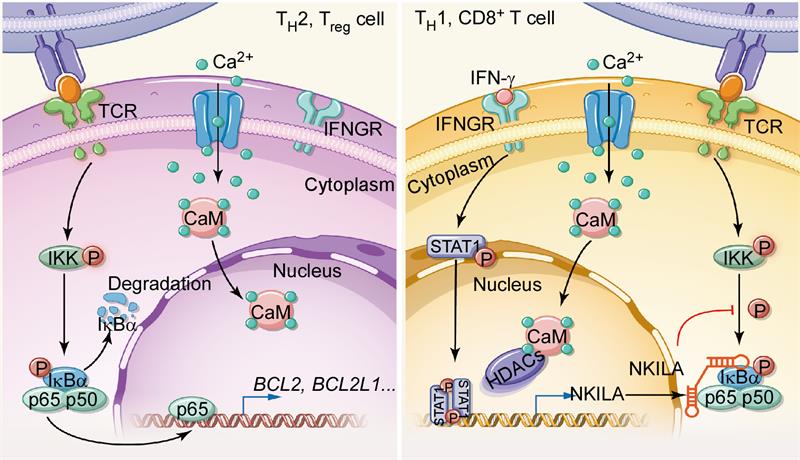
Preferential accumulation of immunosuppressive Treg and TH2 cells, rather than antitumor CTLs and TH1 cells, plays a critical role in tumor immune evasion. Previous studies have ascribed this phenomenon to the favorable conversion of naive T cells to Treg and TH2 cells and to the enhanced recruitment and proliferation of immunosuppressive T cells. Besides, the other mechanism is largely unknown. This paper began by analysing the apoptosis of tumor-infiltrating T cell subsets in cancer patients and noted that higher proportions of tumor-specific CTLs and TH1 cells were apoptotic, compared with Treg cells and TH2 cells. Indeed, when cocultured with autologous tumor cells or FasL, tumor-infiltrating CTLs and TH1 cells underwent massive apoptosis, whereas Treg cells and TH2 cells did not. But the expression level of death receptors were comparably expressed in different T cell subsets. LncRNA-microarray profiling showed that NKILA was significantly elevated in apoptosis-sensitive T cells. Moreover, NKILA silencing decreased T cell apoptosis and enhanced the cytotoxicity of tumour-antigen-activated CTLs towards target tumor cells. Similarly, NKILA also plays an important role in regulating the sensitivity to tumor-mediated apoptosis of different T cell subsets. Mechanistically, in resting T cells, histone deacetylases (HDACs) occupy the NKILA promoter and block STAT1 access, but following TCR stimulation, activated calmodulin translocates into nuclear and removes HDACs from the NKILA promoter to allow STAT1-mediated NKILA transcription. CTLs and TH1 cells with high STAT1 activities have higher levels of NKILA, which binds to NF-κB/IκB, and directly masks phosphorylation motifs of IκB, thereby inhibiting NF-κB activation and the transcription of anti-apoptotic genes.
To investigate whether NKILA could be the target used to improve adoptive cell therapy, the authors transferred CD8+ T cells transduced with either NKILA shRNA or an empty vector into immunocompromised mice bearing established human breast cancer patient-derived xenografts (PDX). Adoptive transfer of CTLs with NKILA knockdown efficiently inhibited tumour growth by increasing CTL infiltration and enhancing their anti-tumor immunity.
To conclude, this study shows for the first time that targeting an lncRNA to protect anti-tumor T cells from tumor-mediated apoptosis could be a feasible approach to improve adoptive T cell therapy.
Full text link: https://www.nature.com/articles/s41590-018-0207-y
Previous studies have demonstrated that immune surveillance is responsible for recognizing and eliminating the tumors, but tumors have acquired the ability to circumvent immune recognition and/or destruction through many different mechanisms. To overcome tumor immune escape, the most well known treatment is cancer immunotherapy, including immune checkpoint inhibitors and adoptive cell transfer therapy. Cancer immunotherapy was picked as the outstanding scientific achievement by Science’s top 10 breakthroughs of year 2013 and 2017, and won the 2018 Nobel Prize in Physiology or Medicine, which has been proven effective against hard-to-treat lymphoma and melanoma. However, immunotherapy is less effective in solid cancer, suggesting that the underlying mechanisms need further investigation.
Preferential accumulation of immunosuppressive Treg and TH2 cells, rather than antitumor CTLs and TH1 cells, plays a critical role in tumor immune evasion. Previous studies have ascribed this phenomenon to the favorable conversion of naive T cells to Treg and TH2 cells and to the enhanced recruitment and proliferation of immunosuppressive T cells. Besides, the other mechanism is largely unknown. This paper began by analysing the apoptosis of tumor-infiltrating T cell subsets in cancer patients and noted that higher proportions of tumor-specific CTLs and TH1 cells were apoptotic, compared with Treg cells and TH2 cells. Indeed, when cocultured with autologous tumor cells or FasL, tumor-infiltrating CTLs and TH1 cells underwent massive apoptosis, whereas Treg cells and TH2 cells did not. But the expression level of death receptors were comparably expressed in different T cell subsets. LncRNA-microarray profiling showed that NKILA was significantly elevated in apoptosis-sensitive T cells. Moreover, NKILA silencing decreased T cell apoptosis and enhanced the cytotoxicity of tumour-antigen-activated CTLs towards target tumor cells. Similarly, NKILA also plays an important role in regulating the sensitivity to tumor-mediated apoptosis of different T cell subsets. Mechanistically, in resting T cells, histone deacetylases (HDACs) occupy the NKILA promoter and block STAT1 access, but following TCR stimulation, activated calmodulin translocates into nuclear and removes HDACs from the NKILA promoter to allow STAT1-mediated NKILA transcription. CTLs and TH1 cells with high STAT1 activities have higher levels of NKILA, which binds to NF-κB/IκB, and directly masks phosphorylation motifs of IκB, thereby inhibiting NF-κB activation and the transcription of anti-apoptotic genes.

The scheme of the mechanism underlying NKILA expression and contribution to the sensitivity to AICD of different T cell subsets
To investigate whether NKILA could be the target used to improve adoptive cell therapy, the authors transferred CD8+ T cells transduced with either NKILA shRNA or an empty vector into immunocompromised mice bearing established human breast cancer patient-derived xenografts (PDX). Adoptive transfer of CTLs with NKILA knockdown efficiently inhibited tumour growth by increasing CTL infiltration and enhancing their anti-tumor immunity.
To conclude, this study shows for the first time that targeting an lncRNA to protect anti-tumor T cells from tumor-mediated apoptosis could be a feasible approach to improve adoptive T cell therapy.
Full text link: https://www.nature.com/articles/s41590-018-0207-y


