Chinese Scholars Unveil New Mechanisms Responsible for the Synergistic Effect of ICIs and Therapeutic Antibodies
Source:Erwei Song
2018-10-16
Monoclonal antibody is one of the most important agents of target therapy for various malignancies. Interaction between antibody Fc fragment and FcgRs eliminates antibody-bound tumor cells via antibody-dependent cellular cytotoxicity (ADCC) mainly by natural killer (NK) cells. ADCC has been studied extensively and is critical for the tumoricidal effects of therapeutic antibodies. FcgRs on macrophages mediate antibody-dependent cellular phagocytosis (ADCP). It was suggested that ADCP is tumoricidal as macrophages phagocytose antibody-bound tumor cells. Although the role of ADCC is well documented, much less is known about the exact phenotype and function of ADCP-macrophages, let alone the overall effect of ADCP on monoclonal antibody therapy.
Prof. Erwei Song, Prof. Qiang Liu and their colleagues from Sun Yat-sen Memorial Hospital of Sun Yat-sen University, demonstrated that monoclonal antibody-mediated tumor cell phagocytosis converts macrophages into an immunosuppressive phenotype. The studies unveil the underlying mechanism and propose a combined strategy of immune therapy based on preclinical evidences. The study with the title “Immune Checkpoint Inhibition Overcomes ADCP-induced Immunosuppression by Macrophages” was published in Cell Journal on October 4th.
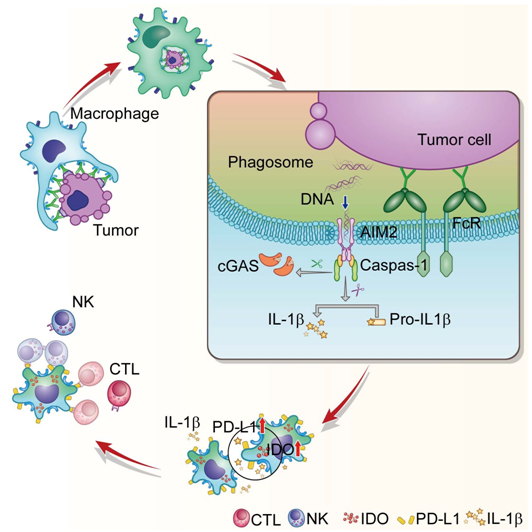
The highlights of their findings are as follows: In ADCP-macrophages, DNA sensor AIM2 is recruited to the phagosomes specifically by FcgR signaling and activated by sensing the phagocytosed tumor DNAs through the disrupted phagosomal membrane. Inflammasome-activated caspase-1 antagonizes IRF-IFN pathway by cleaving cGAS, and up-regulates PD-L1 and IDOs by converting pro-IL-1β into bioactive form. PD-L1 and IDO inhibit NK cell-mediated ADCC and T cell-mediated adaptive immune response, which subsequently hamper therapeutic efficacy of monoclonal antibodies.
Evaluate Trastuzumab-mediated immune responses in an immunocompetent mouse model
To investigate the efficacy of anti-Her2 antibodies in the immunocompetent mice, they used a syngeneic mouse breast cancer cell line, E0771. Both Trastuzumab and its parental drug 4D5 are anti-human Her2 antibodies. Therefore, they ectopically expressed human her2 in E0771 cells. However, human Her2+ synergic graft can not grow up in wild-type mice, which are immune intolerant to human Her2. To circumvent this problem, they used a huErbB2 knock-in mouse model with human Her2 immune tolerance. To further investigate the role of macrophages in this model, they crossed huErbB2 knock-in mice with Csf1-deficient mice, in which macrophages were depleted due to the loss of the major lineage regulator. Surprisingly, enhanced efficacy of 4D5 accompanied with remarkably increased infiltration of NK and CTL cells in the tumors were observed in macrophage-deficient mice, indicating ADCP-macrophages induce immunosuppression, rather than immune-activation. Consistently, in vitro experiments confirmed that human macrophages undergoing ADCP inhibit NK cell-mediated ADCC and T cell-mediated immune response.
Further mechanistic studies demonstrated that DNA sensor AIM2 detects tumor DNA (both gDNA and mtDNA) in phagosomes through their disrupted membrane. Caspase-1, activated by AIM2-inflammasome, cleaves IL-1β into bioactive form. PD-L1 and IDOs in ADCP-macrophages induced by IL-1β inhibit NK cell-mediated ADCC and T cell-mediated cytotoxicity.

How DNA sensors are selectively activated in tumor DNA detection.
Pattern-recognition receptors of innate immune cells can recognize pathogenic nucleic acids and thus initiate host antimicrobial responses. Recent studies have shown that cytosolic sensors in innate immune cells can also detect nucleic acids from tumor cells and trigger anti-tumor immune response. For example, tumor-derived DNA taken up by dendritic cells can activate the cGAS-STING pathway, which induces type I interferon secretion and leads to adaptive T cell response. Their study advances the knowledge in this emerging field by showing that another cytosolic DNA sensor, AIM2, interacts with tumor DNA released from the phagosomes of macrophages after ADCP. Instead of activating anti-tumor immunity, AIM2 inflammasome activates IL-1β and up-regulates PD-L1 and IDO in the macrophages. Interestingly, their data demonstrates that cGAS-STING pathway is not activated in ADCP-macrophages. Why the tumor DNA activates AIM2, rather than cGAS in this scenario? Their data demonstrate that antibody-bound-FcRs in the phagosomal membrane selectively recruits AIM2 to phagosomes via Syk. AIMs, which is prioritized to detect phagocytosed tumor DNA, cleaves cGAS by caspase-1 and inhibits downstream IRF-IFN pathway. Collectively, these data indicate that the specific recruitment and reciprocal antagonism play crucial roles in the selective activation of DNA sensors during tumor DNA detection.
A rationale to combine monoclonal antibodies with ICIs even though the ICs are low in the treatment-naïve tumors.
To investigate the expression of immune checkpoints before and after monoclonal antibody treatments in human, they evaluated the paired pre-treatment biopsies and post-treatment resected samples from HER2+ patients who received neoadjuvant trastuzumab-based therapy. They observed only modest levels of PD-L1 and IDOs in the treatment-naïve Her2 tumors. However, tumor-infiltrating macrophages, but not tumor cells, expressed considerably higher levels of PD-L1 and IDOs after Trastuzumab-containing neoadjuvant therapy. In mice, administration of anti-PD-L1 or/and IDO inhibitor had no appreciable effects on HER2+ tumor growth. However, addition of either anti-PD-L1 or IDO inhibitor significantly improved the response of HER2+ tumors to anti-Her2 antibodies. Importantly, this synergistic effect was abolished in macrophage-deficient mice. PD-L1 expression is an important indicator of anti-PD-1/PD-L1 treatment for cancer patients, and it has been shown that PD-L1 expression in tumor-infiltrating immune cells, particularly macrophages, is more closely associated with clinical responses to anti-PD-L1 treatment than PD-L1 expression in tumor cells in various types of malignancies. Previous clinical trials of ICIs in breast cancer were mostly restricted to the triple negative subtype because the expression of immune checkpoints was usually low in other subtypes. Although PD-L1 and IDO are not abundantly expressed in HER2+ breast cancer before treatment, their data demonstrated that PD-L1 and IDO overexpression was induced in macrophages after neoadjuvant Trastuzumab therapy. These results suggest that it is feasible to combine therapeutic antibodies with ICIs in tumors, even whose immune checkpoint expressions are low in the treatment-naïve state, and the number of tumor-infiltrating macrophages is likely the biomarker to predict the efficacy of this combined therapy.
Prof. Erwei Song, Prof. Qiang Liu and their colleagues from Sun Yat-sen Memorial Hospital of Sun Yat-sen University, demonstrated that monoclonal antibody-mediated tumor cell phagocytosis converts macrophages into an immunosuppressive phenotype. The studies unveil the underlying mechanism and propose a combined strategy of immune therapy based on preclinical evidences. The study with the title “Immune Checkpoint Inhibition Overcomes ADCP-induced Immunosuppression by Macrophages” was published in Cell Journal on October 4th.
The highlights of their findings are as follows: In ADCP-macrophages, DNA sensor AIM2 is recruited to the phagosomes specifically by FcgR signaling and activated by sensing the phagocytosed tumor DNAs through the disrupted phagosomal membrane. Inflammasome-activated caspase-1 antagonizes IRF-IFN pathway by cleaving cGAS, and up-regulates PD-L1 and IDOs by converting pro-IL-1β into bioactive form. PD-L1 and IDO inhibit NK cell-mediated ADCC and T cell-mediated adaptive immune response, which subsequently hamper therapeutic efficacy of monoclonal antibodies.
Evaluate Trastuzumab-mediated immune responses in an immunocompetent mouse model
To investigate the efficacy of anti-Her2 antibodies in the immunocompetent mice, they used a syngeneic mouse breast cancer cell line, E0771. Both Trastuzumab and its parental drug 4D5 are anti-human Her2 antibodies. Therefore, they ectopically expressed human her2 in E0771 cells. However, human Her2+ synergic graft can not grow up in wild-type mice, which are immune intolerant to human Her2. To circumvent this problem, they used a huErbB2 knock-in mouse model with human Her2 immune tolerance. To further investigate the role of macrophages in this model, they crossed huErbB2 knock-in mice with Csf1-deficient mice, in which macrophages were depleted due to the loss of the major lineage regulator. Surprisingly, enhanced efficacy of 4D5 accompanied with remarkably increased infiltration of NK and CTL cells in the tumors were observed in macrophage-deficient mice, indicating ADCP-macrophages induce immunosuppression, rather than immune-activation. Consistently, in vitro experiments confirmed that human macrophages undergoing ADCP inhibit NK cell-mediated ADCC and T cell-mediated immune response.
Further mechanistic studies demonstrated that DNA sensor AIM2 detects tumor DNA (both gDNA and mtDNA) in phagosomes through their disrupted membrane. Caspase-1, activated by AIM2-inflammasome, cleaves IL-1β into bioactive form. PD-L1 and IDOs in ADCP-macrophages induced by IL-1β inhibit NK cell-mediated ADCC and T cell-mediated cytotoxicity.

How DNA sensors are selectively activated in tumor DNA detection.
Pattern-recognition receptors of innate immune cells can recognize pathogenic nucleic acids and thus initiate host antimicrobial responses. Recent studies have shown that cytosolic sensors in innate immune cells can also detect nucleic acids from tumor cells and trigger anti-tumor immune response. For example, tumor-derived DNA taken up by dendritic cells can activate the cGAS-STING pathway, which induces type I interferon secretion and leads to adaptive T cell response. Their study advances the knowledge in this emerging field by showing that another cytosolic DNA sensor, AIM2, interacts with tumor DNA released from the phagosomes of macrophages after ADCP. Instead of activating anti-tumor immunity, AIM2 inflammasome activates IL-1β and up-regulates PD-L1 and IDO in the macrophages. Interestingly, their data demonstrates that cGAS-STING pathway is not activated in ADCP-macrophages. Why the tumor DNA activates AIM2, rather than cGAS in this scenario? Their data demonstrate that antibody-bound-FcRs in the phagosomal membrane selectively recruits AIM2 to phagosomes via Syk. AIMs, which is prioritized to detect phagocytosed tumor DNA, cleaves cGAS by caspase-1 and inhibits downstream IRF-IFN pathway. Collectively, these data indicate that the specific recruitment and reciprocal antagonism play crucial roles in the selective activation of DNA sensors during tumor DNA detection.
A rationale to combine monoclonal antibodies with ICIs even though the ICs are low in the treatment-naïve tumors.
To investigate the expression of immune checkpoints before and after monoclonal antibody treatments in human, they evaluated the paired pre-treatment biopsies and post-treatment resected samples from HER2+ patients who received neoadjuvant trastuzumab-based therapy. They observed only modest levels of PD-L1 and IDOs in the treatment-naïve Her2 tumors. However, tumor-infiltrating macrophages, but not tumor cells, expressed considerably higher levels of PD-L1 and IDOs after Trastuzumab-containing neoadjuvant therapy. In mice, administration of anti-PD-L1 or/and IDO inhibitor had no appreciable effects on HER2+ tumor growth. However, addition of either anti-PD-L1 or IDO inhibitor significantly improved the response of HER2+ tumors to anti-Her2 antibodies. Importantly, this synergistic effect was abolished in macrophage-deficient mice. PD-L1 expression is an important indicator of anti-PD-1/PD-L1 treatment for cancer patients, and it has been shown that PD-L1 expression in tumor-infiltrating immune cells, particularly macrophages, is more closely associated with clinical responses to anti-PD-L1 treatment than PD-L1 expression in tumor cells in various types of malignancies. Previous clinical trials of ICIs in breast cancer were mostly restricted to the triple negative subtype because the expression of immune checkpoints was usually low in other subtypes. Although PD-L1 and IDO are not abundantly expressed in HER2+ breast cancer before treatment, their data demonstrated that PD-L1 and IDO overexpression was induced in macrophages after neoadjuvant Trastuzumab therapy. These results suggest that it is feasible to combine therapeutic antibodies with ICIs in tumors, even whose immune checkpoint expressions are low in the treatment-naïve state, and the number of tumor-infiltrating macrophages is likely the biomarker to predict the efficacy of this combined therapy.



