Prof. Di Wang’s group revealed a new mechanism of intermodulation between immune system and metabolism
Source:Di Wang
2018-12-06
On October 23rd, Professor Di Wang’s group from Zhejiang University published a paper in Immunity entitled “Cholesterol Homeostatic Regulator SCAP-SREBP2 Integrates NLRP3 Inflammasome Activation and Cholesterol Biosynthetic Signaling in Macrophages”. This study reveals that cholesterol biosynthetic master regulator SCAP-SREBP2 promoted NLRP3 inflammasome activation during inflammation, integrating cholesterol metabolism with inflammation in macrophages.
Immune cells display varying metabolic profiles to fulfill their functions. For instance, M1-like macrophages and effector T cells prefer glycolysis to support their inflammatory function, while M2-like macrophages and memory T cells take advantage of fatty acid oxidation for their long-term survival. Cholesterol metabolism has been linked to immune functions. Excess cholesterol can form cholesterol crystals, which directly activate the NLRP3 inflammasome and underlie the pathogenesis of atherosclerosis. However, the mechanisms by which cholesterol biosynthetic signaling orchestrates inflammasome activation remain unclear.
Using a series of cytobiological and biochemical methods, high resolution confocal microscopy, combined with mice disease models, the researchers found that NLRP3 inflammasome activation is integrated with the maturation of cholesterol master transcription factor SREBP2 and endoplasmic reticulum-to-Golgi translocation of SCAP-SREBP2 complex. Importantly, inhibiting this translocation and knockout of SCAP or SREBP2 can both significantly inhibit NLRP3 inflammasome activation, while enforced cholesterol biosynthetic signaling by sterol depletion or statins promoted NLPR3 inflammasome activation. However, this regulation did not predominantly depend on changes in cholesterol homeostasis controlled by the transcriptional activity of SREBP2, as different concentrations of cholesterol cannot rescue this regulation, but relied on the escort activity of SCAP. Mechanistically, NLRP3 associated with SCAP-SREBP2 to form a ternary complex which translocated to the Golgi apparatus adjacent to a mitochondrial cluster for optimal inflammasome assembly.
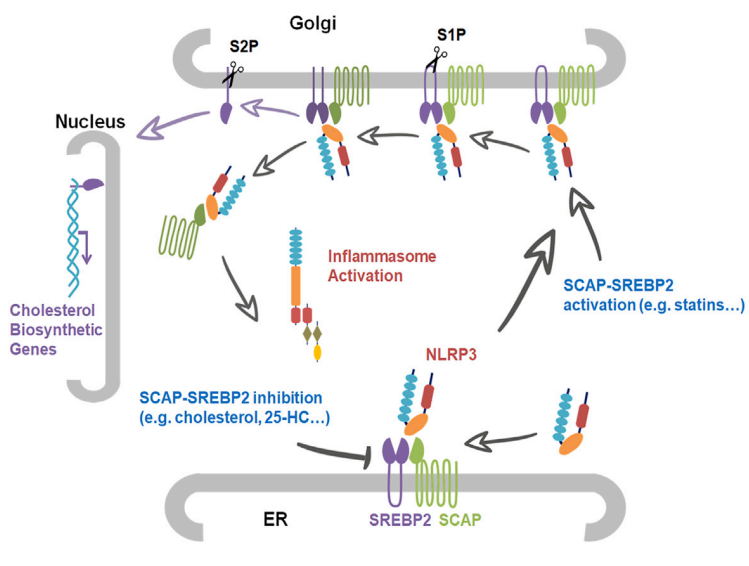
These results strongly support the idea that SCAP-SREBP2 promotes NLRP3 inflammasome activation mainly in a direct manner without affecting cholesterol homeostasis. However, this raises the question of the physiological purpose of the increased cholesterol biosynthetic signaling during NLRP3 inflammasome activation, which was not addressed in this study. Recently, an increasing body of evidence has suggested that the inflammasome-mediated cytokine maturation and pyroptosis are not tightly coupled. The researchers propose that the ‘‘activated’’ macrophages may have a mechanism that senses the severity of a threat to determine whether cell death is obligatory for defense. Otherwise, if secreted cytokines are capable of relieving the threat, SCAP-SREBP complex-mediated lipogenesis could preserve the integrity of the plasma membrane to promote cell survival. Experimental evidence also showed that cholesterol at physiological concentration had potent inhibitory effects on activation stage of the NLRP3 inflammasome, supporting its regulation role in immune response.
Statins are widely used drugs to lower cholesterol levels in clinic, which slow the progression of atherosclerosis, and reduce morbidity and mortality of coronary heart disease. Even so, they have been associated with increased risk of additional-onset diabetes and myopathy, suggesting that moderate levels of cholesterol are essential for the homeostasis of the whole body. In this study, mice fed a statin diet showed higher IL-1β level in plasma after LPS challenge compared to normal chow fed mice. Therefore, this research may serve as one possible explanation for the side effects of statins. It will be worthwhile to examine their effects on inflammasome activation in human subjects; this might foster additional therapeutic and diagnostic strategies for statin-induced risk of disease.
Chuansheng Guo, a postdoc fellow, Zhexu Chi and Danlu Jiang, PhD students are co-first authors of the paper. Prof. Baoliang Song from Wuhan University is the main collaborate of this study. Prof. Linrong Lu from Zhejiang University, Prof. Yingliang Wu and Prof. Wenxin Li from Wuhan University also participated in the study. This work was supported by the National Natural Science Foundation of China, the National Key Research and Development Program of China, and Fundamental Research Funds for the Central Universities.
Immune cells display varying metabolic profiles to fulfill their functions. For instance, M1-like macrophages and effector T cells prefer glycolysis to support their inflammatory function, while M2-like macrophages and memory T cells take advantage of fatty acid oxidation for their long-term survival. Cholesterol metabolism has been linked to immune functions. Excess cholesterol can form cholesterol crystals, which directly activate the NLRP3 inflammasome and underlie the pathogenesis of atherosclerosis. However, the mechanisms by which cholesterol biosynthetic signaling orchestrates inflammasome activation remain unclear.
Using a series of cytobiological and biochemical methods, high resolution confocal microscopy, combined with mice disease models, the researchers found that NLRP3 inflammasome activation is integrated with the maturation of cholesterol master transcription factor SREBP2 and endoplasmic reticulum-to-Golgi translocation of SCAP-SREBP2 complex. Importantly, inhibiting this translocation and knockout of SCAP or SREBP2 can both significantly inhibit NLRP3 inflammasome activation, while enforced cholesterol biosynthetic signaling by sterol depletion or statins promoted NLPR3 inflammasome activation. However, this regulation did not predominantly depend on changes in cholesterol homeostasis controlled by the transcriptional activity of SREBP2, as different concentrations of cholesterol cannot rescue this regulation, but relied on the escort activity of SCAP. Mechanistically, NLRP3 associated with SCAP-SREBP2 to form a ternary complex which translocated to the Golgi apparatus adjacent to a mitochondrial cluster for optimal inflammasome assembly.

These results strongly support the idea that SCAP-SREBP2 promotes NLRP3 inflammasome activation mainly in a direct manner without affecting cholesterol homeostasis. However, this raises the question of the physiological purpose of the increased cholesterol biosynthetic signaling during NLRP3 inflammasome activation, which was not addressed in this study. Recently, an increasing body of evidence has suggested that the inflammasome-mediated cytokine maturation and pyroptosis are not tightly coupled. The researchers propose that the ‘‘activated’’ macrophages may have a mechanism that senses the severity of a threat to determine whether cell death is obligatory for defense. Otherwise, if secreted cytokines are capable of relieving the threat, SCAP-SREBP complex-mediated lipogenesis could preserve the integrity of the plasma membrane to promote cell survival. Experimental evidence also showed that cholesterol at physiological concentration had potent inhibitory effects on activation stage of the NLRP3 inflammasome, supporting its regulation role in immune response.
Statins are widely used drugs to lower cholesterol levels in clinic, which slow the progression of atherosclerosis, and reduce morbidity and mortality of coronary heart disease. Even so, they have been associated with increased risk of additional-onset diabetes and myopathy, suggesting that moderate levels of cholesterol are essential for the homeostasis of the whole body. In this study, mice fed a statin diet showed higher IL-1β level in plasma after LPS challenge compared to normal chow fed mice. Therefore, this research may serve as one possible explanation for the side effects of statins. It will be worthwhile to examine their effects on inflammasome activation in human subjects; this might foster additional therapeutic and diagnostic strategies for statin-induced risk of disease.
Chuansheng Guo, a postdoc fellow, Zhexu Chi and Danlu Jiang, PhD students are co-first authors of the paper. Prof. Baoliang Song from Wuhan University is the main collaborate of this study. Prof. Linrong Lu from Zhejiang University, Prof. Yingliang Wu and Prof. Wenxin Li from Wuhan University also participated in the study. This work was supported by the National Natural Science Foundation of China, the National Key Research and Development Program of China, and Fundamental Research Funds for the Central Universities.


