Prof. Xue-Min Zhang and Tao Li revealed that G3BP1 is critical for the DNA-binding and activation of cGAS
Source:Tao Li
2019-01-04
The research groups directed by Professor Xue-Min Zhang and Tao Li recently published an article in Nature Immunology, entitled “G3BP1 promotes DNA binding and activation of cGAS”. They identified G3BP1 as a critical regulator of cGAS activity, which plays an essential role in cGAS-mediated innate immune responses.
Pathogen infection introduces a series of “non-self” signals to the host, one of which is the emergence of DNA in cytoplasm. Sensing of cytosolic DNA by the key DNA sensor cGAS leads to the activation of innate immune response and is crucial for the elimination of pathogens. However, the aberrant activation of cGAS by self-DNA has been implicated in several autoimmune diseases such as Aicardi–Goutières syndrome (AGS). Thus, understanding the regulation of cGAS activity will provide new insight into both the inhibition of viral infection and the treatment of autoimmune diseases.
The researchers first identified G3BP1 as one of the cGAS-interacting proteins. By employing G3BP1−/− U937 cells (human monocytic cell line) and G3bp1−/− primary mouse embryo fibroblasts (MEFs), they found that deletion of G3BP1 appreciably affected the production of type I IFN in response to different types of intracellular DNAs, indicating the critical role of G3BP1 in cGAS-mediated immune responses. The researchers further found that depletion of G3BP1 significantly reduced cGAMP production, while it did not inhibit cGAMP-induced downstream signaling. These results suggested that G3BP1 regulates cGAS/STING pathway by regulating cGAS activity. Through biochemical assays including in vitro cGAMP synthesis assays and EMSAs, it was demonstrated that G3BP1 ensures efficient DNA-binding and activation of cGAS by promoting the oligomerization of cGAS.
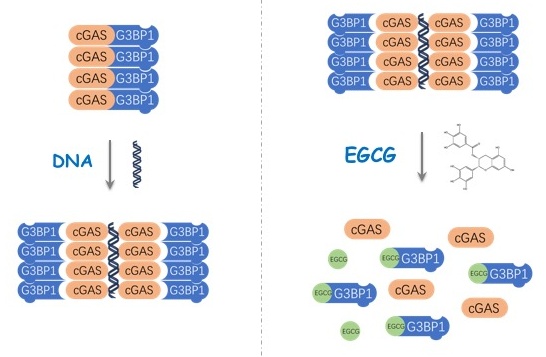
Based on these findings, the researchers also found that EGCG, which is a natural chemical from green tea and a known inhibitor of G3BP1, could efficiently inhibit cGAS activity through disrupting G3BP1-cGAS complexes. By using Trex1−/− mice and AGS patient cells, they further demonstrated the efficacy of EGCG in treating cGAS-mediated autoinflammatory responses.
This study identified a pivotal regulator for cGAS activity, and provided a tangible treatment for cGAS-related autoimmune diseases.
Pathogen infection introduces a series of “non-self” signals to the host, one of which is the emergence of DNA in cytoplasm. Sensing of cytosolic DNA by the key DNA sensor cGAS leads to the activation of innate immune response and is crucial for the elimination of pathogens. However, the aberrant activation of cGAS by self-DNA has been implicated in several autoimmune diseases such as Aicardi–Goutières syndrome (AGS). Thus, understanding the regulation of cGAS activity will provide new insight into both the inhibition of viral infection and the treatment of autoimmune diseases.
The researchers first identified G3BP1 as one of the cGAS-interacting proteins. By employing G3BP1−/− U937 cells (human monocytic cell line) and G3bp1−/− primary mouse embryo fibroblasts (MEFs), they found that deletion of G3BP1 appreciably affected the production of type I IFN in response to different types of intracellular DNAs, indicating the critical role of G3BP1 in cGAS-mediated immune responses. The researchers further found that depletion of G3BP1 significantly reduced cGAMP production, while it did not inhibit cGAMP-induced downstream signaling. These results suggested that G3BP1 regulates cGAS/STING pathway by regulating cGAS activity. Through biochemical assays including in vitro cGAMP synthesis assays and EMSAs, it was demonstrated that G3BP1 ensures efficient DNA-binding and activation of cGAS by promoting the oligomerization of cGAS.

Based on these findings, the researchers also found that EGCG, which is a natural chemical from green tea and a known inhibitor of G3BP1, could efficiently inhibit cGAS activity through disrupting G3BP1-cGAS complexes. By using Trex1−/− mice and AGS patient cells, they further demonstrated the efficacy of EGCG in treating cGAS-mediated autoinflammatory responses.
This study identified a pivotal regulator for cGAS activity, and provided a tangible treatment for cGAS-related autoimmune diseases.


