Dr. Wei Zhao’s group reveals that redox homeostasis maintained by GPX4 facilitates STING activation
Source:Wei Zhao
2020-07-10
The DNA sensor cGAS recognizes cytosolic pathogen-derived DNA (such as viral DNA) or self-DNA from genomic DNA damage and catalyzes the synthesis of the second messenger cGAMP, which binds to the adapter protein STING. Binding of cGAMP triggers STING trafficking from the ER to the Golgi apparatus, where it recruits TBK1 and IRF3, which results in the initiating innate immune response. Redox homeostasis is a vital component of a physiological cellular steady-state. Impaired redox homeostasis, such as imbalances in lipid peroxide abundance, is associated with multiple pathological conditions, including viral diseases and cancer-. The lipid hydroperoxidase GPX4 is a unique enzyme that protects cells against membrane lipid peroxidation and maintains redox homeostasis by reducing highly reactive lipid hydroperoxides (LOOH) to non-reactive lipid alcohols. The way in which intracellular redox homeostasis maintained by GPX4 controls STING activation remains unknown.
On June 15, 2020, Wei Zhao’s group of Shandong University published the results of Redox Homeostasis Maintained by GPX4 Facilitates STING Activation, revealing for a new mechanism for the control of STING activity and links cellular redox homeostasis to the DNA-sensing pathway, which provides new insights for innate immunity.
Wei Zhao’s group show that the anti-oxidant enzyme GPX4 is indispensable for the activation of the cGAS-STING pathway by suppression of cellular membrane lipid peroxidation, but not RLR- or TLR- pathway. Lipid peroxidation has a role in homeostasis, as well as in response to different stress. Viral infection induced the cellular level of the end products of lipid peroxidation (such as 4-HNE), and GPX4 deficiency enhances this response more. 4-HNE cause STING carbonylation at the essential residue of C88, which results in the inhibition of STING trafficking from the ER to the Golgi and suppression of STING activation.
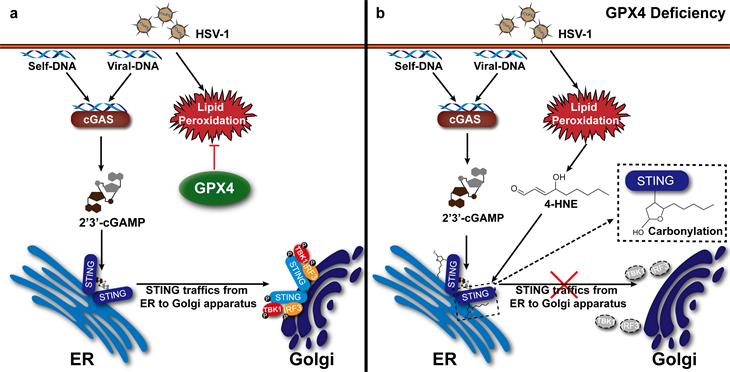
In summary, Wei Zhao’s group uncovers a new mechanism for the control of STING activity and links cellular redox homeostasis to the DNA-sensing pathway, and illustrates a new strategy to regulate STING activation by modulating GPX4 activity and lipid peroxidation, thereby providing a promising therapeutic target for the intervention of diseases with improper STING activation.
Wei Zhao of Shandong University is the corresponding author in this article, and Mutian Jia in the laboratory is the first author. This work was supported by grants from the National Natural Science Foundation of China, the National Key Research and Developmental Program of China and the Newton Advanced Fellowship of the Academy of Medical Sciences.
Links: https://www.nature.com/articles/s41590-020-0699-0
On June 15, 2020, Wei Zhao’s group of Shandong University published the results of Redox Homeostasis Maintained by GPX4 Facilitates STING Activation, revealing for a new mechanism for the control of STING activity and links cellular redox homeostasis to the DNA-sensing pathway, which provides new insights for innate immunity.
Wei Zhao’s group show that the anti-oxidant enzyme GPX4 is indispensable for the activation of the cGAS-STING pathway by suppression of cellular membrane lipid peroxidation, but not RLR- or TLR- pathway. Lipid peroxidation has a role in homeostasis, as well as in response to different stress. Viral infection induced the cellular level of the end products of lipid peroxidation (such as 4-HNE), and GPX4 deficiency enhances this response more. 4-HNE cause STING carbonylation at the essential residue of C88, which results in the inhibition of STING trafficking from the ER to the Golgi and suppression of STING activation.

In summary, Wei Zhao’s group uncovers a new mechanism for the control of STING activity and links cellular redox homeostasis to the DNA-sensing pathway, and illustrates a new strategy to regulate STING activation by modulating GPX4 activity and lipid peroxidation, thereby providing a promising therapeutic target for the intervention of diseases with improper STING activation.
Wei Zhao of Shandong University is the corresponding author in this article, and Mutian Jia in the laboratory is the first author. This work was supported by grants from the National Natural Science Foundation of China, the National Key Research and Developmental Program of China and the Newton Advanced Fellowship of the Academy of Medical Sciences.
Links: https://www.nature.com/articles/s41590-020-0699-0


