Dr. Chenqi Xu’s group developed a new strategy of CAR-T cell therapy
Source:Chenqi Xu
2020-09-14
CAR T-cell therapy is a type of adoptive cell therapy to treat cancer. Using genetic engineering, T cells from patients are equipped with chimeric antigen receptor (CAR) to specifically recognize tumor antigen and kill tumor cells. However, CAR-T cell therapy is facing major clinical challenges including cytokine release syndrome and poor persistence. Therefore, it is important to design CAR-T cells with high persistence, little side effects and strong anti-tumor activity.
Recently, Dr. Chenqi Xu’s group at CAS Center for Excellence in Molecular Cell Science, Dr. Catherine Chiulan Wong’s group at Peking University and Dr. Enfu Hui’s group at University California, San Diego developed a new strategy of CAR-T cell therapy, which has reduced cytokine production, enhanced cell persistence and better anti-tumor function than clinical used 28Z CAR-T cells. This study was published in Cell entitled “Multiple Signaling Roles of CD3ε and Its Application in CAR-T Cell Therapy” on July 29.
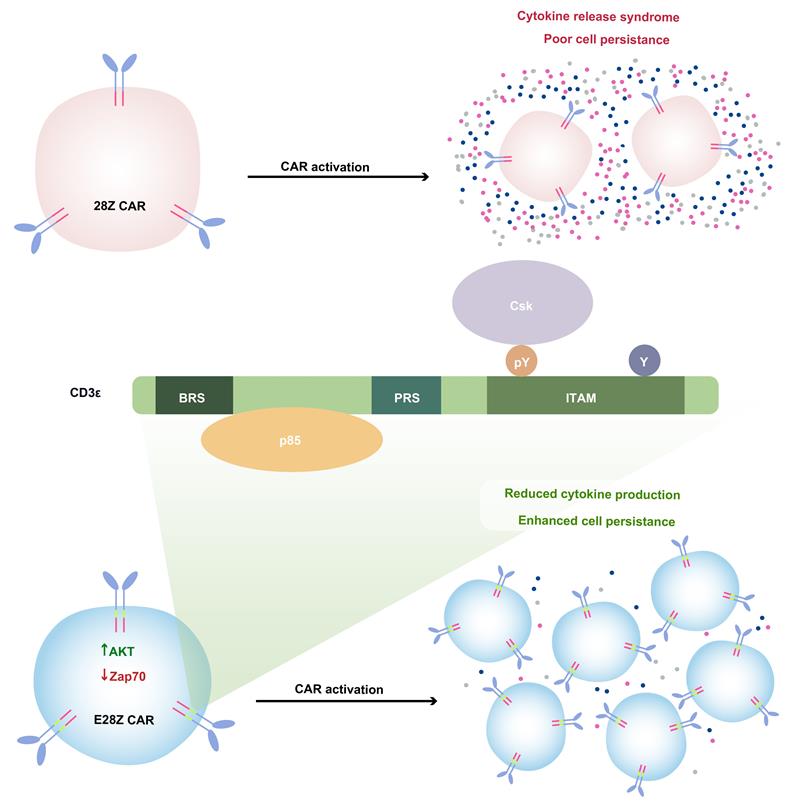
T cell receptor (TCR) mediates antigen-induced signaling through its associated CD3ε, δ, γ, and ζ. Using quantitative mass spectrometry, the authors simultaneously quantitated the phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) of all CD3 chains upon TCR stimulation. The results showed that CD3ε ITAMs was mono-phosphorylated, owing to Lck kinase selectivity, and specifically recruited the inhibitory Csk kinase to attenuate TCR signaling, suggesting that TCR is a self-restrained signaling machinery containing both activating and inhibitory motifs. Next, the authors incorporated CD3ε cytoplasmic domain into the clinical used 28Z CAR, and found that the new E28Z CAR-T cells had improved antitumor activity. Mechanistically, the Csk-recruiting ITAM of CD3ε reduced CAR-T cell cytokine production whereas the basic residue rich sequence (BRS) of CD3ε promoted CAR-T cell persistence via p85 recruitment. Considering the strong anti-tumor activity of E28Z CAR-T cells in both blood tumor and solid tumor models, these results provide a solid rationale to explore its promising translational potentials in treating blood and solid malignancies.
Ph.D. candidates Wei Wu, Qiuping Zhou and Xiaoshan Shi from CAS Center for Excellence in Molecular Cell Science and Dr. Takeya Masubuchi from University California, San Diego are the co-first authors. Dr. Chenqi Xu, Dr. Catherine Chiulan Wong and Dr. Enfu Hui are the co-corresponding authors. Dr. Haopeng Wang from ShanghaiTech University and Dr. Jie Sun from Zhejiang University participate the study. Dr. Chenqi Xu is the adjunct researcher of Hangzhou Institute For Advanced Study of the Chinese Academy of Sciences.
Links: https://www.sciencedirect.com/science/article/pii/S0092867420308837
Recently, Dr. Chenqi Xu’s group at CAS Center for Excellence in Molecular Cell Science, Dr. Catherine Chiulan Wong’s group at Peking University and Dr. Enfu Hui’s group at University California, San Diego developed a new strategy of CAR-T cell therapy, which has reduced cytokine production, enhanced cell persistence and better anti-tumor function than clinical used 28Z CAR-T cells. This study was published in Cell entitled “Multiple Signaling Roles of CD3ε and Its Application in CAR-T Cell Therapy” on July 29.
T cell receptor (TCR) mediates antigen-induced signaling through its associated CD3ε, δ, γ, and ζ. Using quantitative mass spectrometry, the authors simultaneously quantitated the phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) of all CD3 chains upon TCR stimulation. The results showed that CD3ε ITAMs was mono-phosphorylated, owing to Lck kinase selectivity, and specifically recruited the inhibitory Csk kinase to attenuate TCR signaling, suggesting that TCR is a self-restrained signaling machinery containing both activating and inhibitory motifs. Next, the authors incorporated CD3ε cytoplasmic domain into the clinical used 28Z CAR, and found that the new E28Z CAR-T cells had improved antitumor activity. Mechanistically, the Csk-recruiting ITAM of CD3ε reduced CAR-T cell cytokine production whereas the basic residue rich sequence (BRS) of CD3ε promoted CAR-T cell persistence via p85 recruitment. Considering the strong anti-tumor activity of E28Z CAR-T cells in both blood tumor and solid tumor models, these results provide a solid rationale to explore its promising translational potentials in treating blood and solid malignancies.
Ph.D. candidates Wei Wu, Qiuping Zhou and Xiaoshan Shi from CAS Center for Excellence in Molecular Cell Science and Dr. Takeya Masubuchi from University California, San Diego are the co-first authors. Dr. Chenqi Xu, Dr. Catherine Chiulan Wong and Dr. Enfu Hui are the co-corresponding authors. Dr. Haopeng Wang from ShanghaiTech University and Dr. Jie Sun from Zhejiang University participate the study. Dr. Chenqi Xu is the adjunct researcher of Hangzhou Institute For Advanced Study of the Chinese Academy of Sciences.

Clinical used 28Z CAR-T cells are facing major clinical challenges including cytokine release syndrome and poor persistence. E28Z CAR-T cells incorporating the CD3ε cytoplasmic domain have reduced cytokine production due to Csk-recruiting ITAM of CD3ε and enhanced persistence through p85- recruiting BRS of CD3ε.
Links: https://www.sciencedirect.com/science/article/pii/S0092867420308837


