Prof. Erwei Song and Shicheng Su’s Group reveals a novel strategy for promoting T cell trafficking to tumors
Source:Erwei Song
2021-07-06
Tumor immunotherapy is one of the most rapidly developing anti-tumor therapies, but only about 20-30% of patients obtain clinical benefit. Various molecular mechanisms have been linked to the preferential accumulation of pro-tumor lymphocytes, rather than anti-tumor lymphocytes in the tumor stroma. Among them, the immunosuppressive tumor microenvironment (TME) may prime the tumor-infiltrating naive T cells to immunosuppressive T cells. Additionally, anti-tumor T cells are prone to cell death, as they are more sensitive to apoptotic signals in the TME. However, mechanisms underlying the impaired trafficking of tumor-specific TH1 cells and cytotoxic T lymphocytes (CTLs) to tumors remain unclear.
On June 17, 2021, Prof. Erwei Song and Shicheng Su’s group from Sun Yat-Sen Memorial Hospital published an original article on Nature Immunology, entitled “Targeting regulator of G protein signaling 1 in tumor-specific T cells enhances their trafficking to breast cancer”. This study reveals that regulator of G-protein signaling 1 (RGS1), upregulated by IFN-STAT1 signaling in TH1 cells and CTLs, impaired the trafficking of circulating T cells to tumors. RGS1 knockdown in adoptively transferred tumor-specific CTLs significantly increased their infiltration in breast and lung tumor grafts and effectively inhibited tumor growth in vivo, which was further improved when combined with immune checkpoint inhibition against PD-L1.
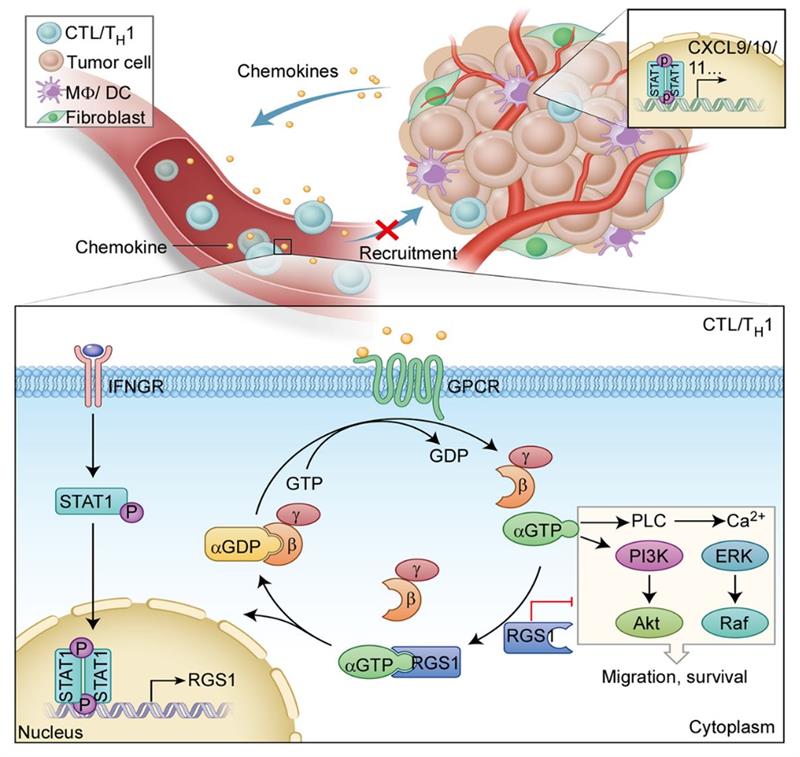
They found that in the TME, reduced infiltration of anti-tumor lymphocytes, such as TH1 cells and CTLs, and abundance infiltration of immunosuppressive TH2 cells correlate with poor cancer survival. One of the most important reason was ascribed to their distinct recruitment to tumor tissues. Further, they found that functional regulation of chemokine receptors by RGS1, rather than their expression or their ligand levels, determines the recruitment capabilities of TH1 cells and CTLs. RGS1 is transcribed by IFNγ-STAT1 signaling in TH1 cells and CTLs and binds to several G-protein-coupled receptors (GPCR). As a GTPase accelerating protein, RGS1 promotes the hydrolysis of GTP to GDP, which deactivates the downstream pathway by inhibiting calcium influx and suppressing the activation of ERK and AKT, thus impairing the circulating T cell homing to tumors. As a result, breast cancers are shown to be ‘cold’ tumors, characterized by less anti-tumor T cell infiltration and more pro-tumor T cell infiltration.
Moreover, they established an adoptive T cell therapy (ACT) model in mice bearing breast or lung tumor grafts. Monitored by intravital two-photon microscopy, Rgs1 knockdown dramatically increases the trafficking and infiltration of transferred CTLs into tumors, and thus effectively suppresses tumor growth. To recapitulate the above findings in a more humanized model, they employed patient-derived xenografts (PDXs) with triple-negative breast cancer in immunocompromised NOD SCID mice. Silencing RGS1 in transferred CTLs significantly improves the trafficking of CTLs to tumors and therefore tremendously enhances the therapeutic effects of ACT, which was further enhanced when combined with anti-PD-L1 inhibitor.
Collectively, this study demonstrates that RGS1 plays a critical role in building an immunosuppressive microenvironment in breast cancer by suppressing the trafficking of TH1 cells and CTLs to the tumor, which may emerge as a new therapeutic target to enhance effector T cell infiltration and turn ‘cold’ breast cancers into hot ones.
Dr. Di Huang, Dr. Xueman Chen, and Dr. Xin Zeng are co-first authors. Academician Erwei Song and Prof. Shicheng Su are co-corresponding authors of this study. This work is supported by grants from the National Key Research and Development Program of China, the Natural Science Foundation of China etc.
Links: https://www.nature.com/articles/s41590-021-00939-9
On June 17, 2021, Prof. Erwei Song and Shicheng Su’s group from Sun Yat-Sen Memorial Hospital published an original article on Nature Immunology, entitled “Targeting regulator of G protein signaling 1 in tumor-specific T cells enhances their trafficking to breast cancer”. This study reveals that regulator of G-protein signaling 1 (RGS1), upregulated by IFN-STAT1 signaling in TH1 cells and CTLs, impaired the trafficking of circulating T cells to tumors. RGS1 knockdown in adoptively transferred tumor-specific CTLs significantly increased their infiltration in breast and lung tumor grafts and effectively inhibited tumor growth in vivo, which was further improved when combined with immune checkpoint inhibition against PD-L1.
They found that in the TME, reduced infiltration of anti-tumor lymphocytes, such as TH1 cells and CTLs, and abundance infiltration of immunosuppressive TH2 cells correlate with poor cancer survival. One of the most important reason was ascribed to their distinct recruitment to tumor tissues. Further, they found that functional regulation of chemokine receptors by RGS1, rather than their expression or their ligand levels, determines the recruitment capabilities of TH1 cells and CTLs. RGS1 is transcribed by IFNγ-STAT1 signaling in TH1 cells and CTLs and binds to several G-protein-coupled receptors (GPCR). As a GTPase accelerating protein, RGS1 promotes the hydrolysis of GTP to GDP, which deactivates the downstream pathway by inhibiting calcium influx and suppressing the activation of ERK and AKT, thus impairing the circulating T cell homing to tumors. As a result, breast cancers are shown to be ‘cold’ tumors, characterized by less anti-tumor T cell infiltration and more pro-tumor T cell infiltration.

Moreover, they established an adoptive T cell therapy (ACT) model in mice bearing breast or lung tumor grafts. Monitored by intravital two-photon microscopy, Rgs1 knockdown dramatically increases the trafficking and infiltration of transferred CTLs into tumors, and thus effectively suppresses tumor growth. To recapitulate the above findings in a more humanized model, they employed patient-derived xenografts (PDXs) with triple-negative breast cancer in immunocompromised NOD SCID mice. Silencing RGS1 in transferred CTLs significantly improves the trafficking of CTLs to tumors and therefore tremendously enhances the therapeutic effects of ACT, which was further enhanced when combined with anti-PD-L1 inhibitor.
Collectively, this study demonstrates that RGS1 plays a critical role in building an immunosuppressive microenvironment in breast cancer by suppressing the trafficking of TH1 cells and CTLs to the tumor, which may emerge as a new therapeutic target to enhance effector T cell infiltration and turn ‘cold’ breast cancers into hot ones.
Dr. Di Huang, Dr. Xueman Chen, and Dr. Xin Zeng are co-first authors. Academician Erwei Song and Prof. Shicheng Su are co-corresponding authors of this study. This work is supported by grants from the National Key Research and Development Program of China, the Natural Science Foundation of China etc.
Links: https://www.nature.com/articles/s41590-021-00939-9


