Prof. Jin Jin and Qian Cao’s group reveals the mechanism of selenium in controlling T cell differentiation in Crohn’s disease
Source:Jin Jin
2021-09-15
On August 2, 2021, Prof. Jin Jin and Dr. Qian Cao’s group from Sir Run Run Shaw Hospital, Zhejiang University, published an original article on Immunity entitled “Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn’s disease.” This study revealed the immunological features and metabolic microenvironment of untreated patients with inflammatory bowel disease (IBD) by multiomics analyses and highlighted selenium as an essential regulator of T cell responses and potential therapeutic targets in Crohn’s disease.
Inflammatory bowel disease (IBD) primarily includes Crohn’s disease (CD) and ulcerative colitis (UC), which are characterized by chronic and relapsing intestinal inflammation. In newly industrialized countries, the incidence of IBD is continuously increasing. The pathogenesis of IBD has not been investigated in depth. Multiple factors, such as genetic mutations, diet, disordered intestinal microbes, and hyperactivation of immunity are involved in the onset of IBD. Among these factors, dysfunction of the mucosal immune system is the most essential in the pathogenesis of CD and UC. However, the individuals included in the previous studies have been administered various medications, which would have affected their pathological immune responses. Thus, the pathological features of immune responses in naive IBD remain poorly investigated. Interestingly, some evidence reveals some differences in the response patterns between the T cells isolated from these two distinct IBD. However, the mechanisms that cause these differences remain poorly investigated. The response pattern of T cells in the intestinal microenvironment is regulated by various factors, including microorganisms, cytokines and chemokines. Recent studies revealed that metabolites are critical mediators in regulating T cell activation, proliferation and differentiation. Selenium is an essential trace mineral incorporated into proteins to form selenoproteins, which play indispensable roles in ROS scavenging and redox regulation. The specific selenoproteins that affect T cell function in IBD remain unclear.
In this work, the authors performed single-cell sequencing of colonic immune cells and metabolomic profiling to clarify the immune microenvironment in the inflammatory area of naïve patients with CD or UC and healthy donors. They demonstrated a series of disease-specific immune cell types and metabolite alterations during the pathogenesis of CD and UC. Remarkably, they discovered unique Th1-like cells expanded in the colon of individuals with naive CD and unique Th17-like cells and exhausted Tc17 cells in individuals with UC. The metabolome study identified 92 and 275 metabolites specifically changed in CD and UC, respectively. An in vitro screening of altered metabolites identified several metabolites that modulate T cell differentiation and reflect the disease-specific pattern of distinct IBDs. Modulation of CD-specific metabolites, particularly reduced selenium, can shape type 1 T helper (Th1) cell differentiation, which was explicitly enriched in CD. Selenium supplementation suppressed Th1 cell differentiation via selenoprotein W (SELW)-mediated cellular reactive oxygen species scavenging. SELW promoted purine salvage pathways and inhibited one-carbon metabolism, which affected the ROS-NF-κB axis and suppressed the production of IFN-γ. Selenium supplementation also alleviates colitis in animal models and patients with CD.
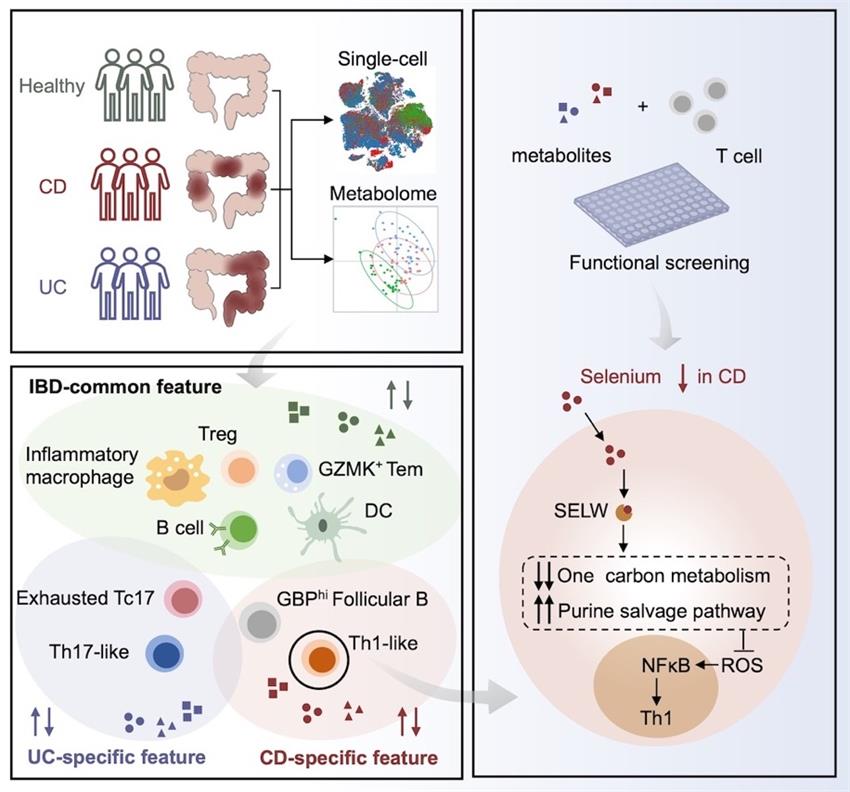
This study comprehensively analyzed the composition and metabolic changes of colonic mucosal immune cells in newly diagnosed CD and UC, and clarified the mechanism of metabolites regulating the immune response pattern of specific disease subtypes. They established selenium deficiency as a pivotal driver in the onset of CD and local Th1 polarization. These results also provide insights into the druggable function of trace elements in regulating adaptive Immunity in inflammatory diseases.
Dr. Jin Jin from the Life Sciences Institute of Zhejiang University and Dr. Qian Cao from Sir Run Run Shaw Hospital, Zhejiang University are the co-corresponding authors. Ph.D. candidates Lingjie Huang, Xintao Mao from Zhejiang University and associate research fellow Yiyuan Li from Southeast University are the co-first authors of this paper. This work was support by the National Key R&D Program of China, Excellent Young Scientist Fund of NSFC, Zhejiang Provincial Natural Science Foundation of China, National Natural Science Foundation of China, and Key R&D Program of Zhejiang Province.
Links: https://doi.org/10.1016/j.immuni.2021.07.004
Inflammatory bowel disease (IBD) primarily includes Crohn’s disease (CD) and ulcerative colitis (UC), which are characterized by chronic and relapsing intestinal inflammation. In newly industrialized countries, the incidence of IBD is continuously increasing. The pathogenesis of IBD has not been investigated in depth. Multiple factors, such as genetic mutations, diet, disordered intestinal microbes, and hyperactivation of immunity are involved in the onset of IBD. Among these factors, dysfunction of the mucosal immune system is the most essential in the pathogenesis of CD and UC. However, the individuals included in the previous studies have been administered various medications, which would have affected their pathological immune responses. Thus, the pathological features of immune responses in naive IBD remain poorly investigated. Interestingly, some evidence reveals some differences in the response patterns between the T cells isolated from these two distinct IBD. However, the mechanisms that cause these differences remain poorly investigated. The response pattern of T cells in the intestinal microenvironment is regulated by various factors, including microorganisms, cytokines and chemokines. Recent studies revealed that metabolites are critical mediators in regulating T cell activation, proliferation and differentiation. Selenium is an essential trace mineral incorporated into proteins to form selenoproteins, which play indispensable roles in ROS scavenging and redox regulation. The specific selenoproteins that affect T cell function in IBD remain unclear.
In this work, the authors performed single-cell sequencing of colonic immune cells and metabolomic profiling to clarify the immune microenvironment in the inflammatory area of naïve patients with CD or UC and healthy donors. They demonstrated a series of disease-specific immune cell types and metabolite alterations during the pathogenesis of CD and UC. Remarkably, they discovered unique Th1-like cells expanded in the colon of individuals with naive CD and unique Th17-like cells and exhausted Tc17 cells in individuals with UC. The metabolome study identified 92 and 275 metabolites specifically changed in CD and UC, respectively. An in vitro screening of altered metabolites identified several metabolites that modulate T cell differentiation and reflect the disease-specific pattern of distinct IBDs. Modulation of CD-specific metabolites, particularly reduced selenium, can shape type 1 T helper (Th1) cell differentiation, which was explicitly enriched in CD. Selenium supplementation suppressed Th1 cell differentiation via selenoprotein W (SELW)-mediated cellular reactive oxygen species scavenging. SELW promoted purine salvage pathways and inhibited one-carbon metabolism, which affected the ROS-NF-κB axis and suppressed the production of IFN-γ. Selenium supplementation also alleviates colitis in animal models and patients with CD.

This study comprehensively analyzed the composition and metabolic changes of colonic mucosal immune cells in newly diagnosed CD and UC, and clarified the mechanism of metabolites regulating the immune response pattern of specific disease subtypes. They established selenium deficiency as a pivotal driver in the onset of CD and local Th1 polarization. These results also provide insights into the druggable function of trace elements in regulating adaptive Immunity in inflammatory diseases.
Dr. Jin Jin from the Life Sciences Institute of Zhejiang University and Dr. Qian Cao from Sir Run Run Shaw Hospital, Zhejiang University are the co-corresponding authors. Ph.D. candidates Lingjie Huang, Xintao Mao from Zhejiang University and associate research fellow Yiyuan Li from Southeast University are the co-first authors of this paper. This work was support by the National Key R&D Program of China, Excellent Young Scientist Fund of NSFC, Zhejiang Provincial Natural Science Foundation of China, National Natural Science Foundation of China, and Key R&D Program of Zhejiang Province.
Links: https://doi.org/10.1016/j.immuni.2021.07.004


