Heterogeneity of human Langerhans cells in development and functions revealed by collaboration of the groups of Xu Yao, Wei Li and Xiao Li
Source:Xiaochun Liu
2021-11-12
Langerhans cells (LCs) are the only antigen presenting cells residing in the normal epidermis, and play a pivotal role in skin homeostasis. LCs are involved in diverse immune responses, either activating immune response, or exerting anti-inflammatory effects by induction of regulatory T cells (Tregs). The heterogeneity of LCs has long been considered, and recent studies have revealed that murine LCs have multiple origins including the yolk sac and monocytes. Moreover, LCs display hybrid properties of both macrophages and dendritic cells in phenotype and function.
On September 10, 2021,Prof. Xu Yao’s group from the Hospital for Skin Diseases, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Prof. Wei Li’s group from Huashan Hospital, Fudan University, and Xiao Li from Texas Heart Institute, Houston, Texas, USA, jointly published an original article entitled “Distinct human Langerhans cell subsets orchestrate reciprocal functions and require different developmental regulation” in Immunity. Their study revealed distinct human LC subsets with unique phenotype and reciprocal functions for the first time, and draw a map of the trajectories of LC subset differentiation.
In this study, the authors identified two steady-state (CD207hiCD1ahiCD1clo/- LC1 and CD207intCD1aintCD1chi LC2) and two activated LC subsets (aLC and migLC) in the epidermis of human skin and in LCs derived from CD34+ hemopoietic stem cells (HSC-LCs) by utilizing single-cell RNA sequencing, mass cytometry and flow cytometry. Further analysis of differential genes and gene ontology (GO) analyses revealed that LC1 were characterized as classical LCs, mainly related to innate immunity and antigen processing. LC2 were similar to monocytes or myeloid dendritic cells, involving in immune responses and leukocyte activation. aLC and migLC, expressing CD83 and CCR7, represented the LC populations that were activated and began to migrate.
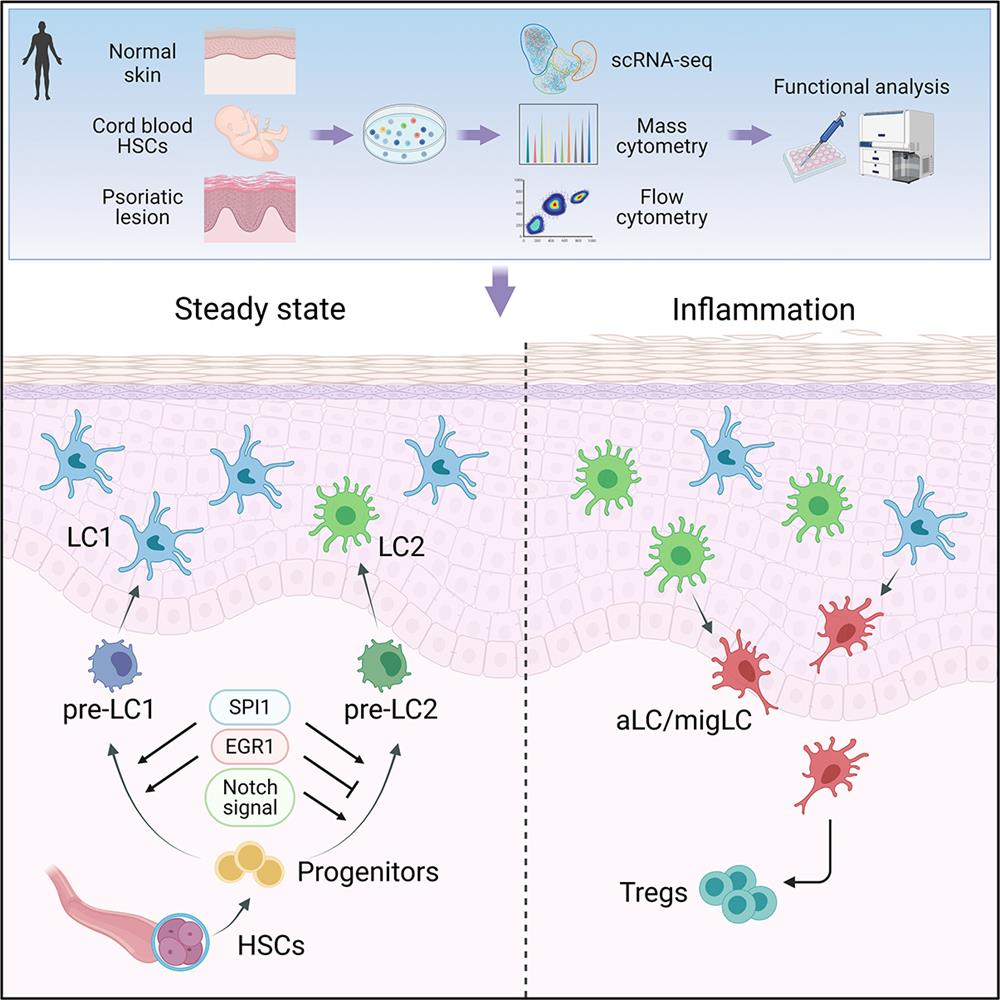
The authors depicted the full differentiation trajectories of LCs by analysis of HSC-LCs at multiple time-points during differentiation. SPI1, ID2, and NF-κB pathway directed LC differentiation and balanced the differentiation and activation of LCs. Pre-LCs gradually transitioned into steady-state LC1 and LC2. LC1 and LC2 branches appeared almost simultaneously during differentiation, indicating relatively independent ontogeny. EGR1 and notch signaling regulated LC1 and LC2 bifurcation.
LC1 and LC2 orchestrated reciprocal functions as revealed by analysis of the scRNA-seq data and the in vitro functional studies. LC1 had superior self-maintenance and capacity for antigen uptake, and remained stable under inflammatory microenvironment. LC2 were prone to being activated and demonstrated elevated expression of immuno-suppressive molecules (PD-L1 and RANK), and were more capable of migration and were likely to assume an immuno-regulatory role. The authors further analyzed the changes of LC subsets in psoriatic lesions. They found that the numbers of LC2 and aLC/migLC in psoriatic skin were increased, and the number of PD-L1+ T cells and CD4+Foxp3+ Treg cells in psoriatic lesions were also increased. The interaction of LCs and T cells might assume an immuno-regulatory role to counterbalance against skin inflammation.
Xiaochun Liu, a resident from Hospital for Skin Diseases, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, and Ronghui Zhu, a PhD student from Huashan Hospital, Fudan University, are the co-first authors. Xiao Liu from Tsinghua Shenzhen International Graduate School, Tsinghua University is the senior author. Prof. Wei Li is the Lead contact.
Links: https://doi.org/10.1016/j.immuni.2021.08.012
On September 10, 2021,Prof. Xu Yao’s group from the Hospital for Skin Diseases, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Prof. Wei Li’s group from Huashan Hospital, Fudan University, and Xiao Li from Texas Heart Institute, Houston, Texas, USA, jointly published an original article entitled “Distinct human Langerhans cell subsets orchestrate reciprocal functions and require different developmental regulation” in Immunity. Their study revealed distinct human LC subsets with unique phenotype and reciprocal functions for the first time, and draw a map of the trajectories of LC subset differentiation.
In this study, the authors identified two steady-state (CD207hiCD1ahiCD1clo/- LC1 and CD207intCD1aintCD1chi LC2) and two activated LC subsets (aLC and migLC) in the epidermis of human skin and in LCs derived from CD34+ hemopoietic stem cells (HSC-LCs) by utilizing single-cell RNA sequencing, mass cytometry and flow cytometry. Further analysis of differential genes and gene ontology (GO) analyses revealed that LC1 were characterized as classical LCs, mainly related to innate immunity and antigen processing. LC2 were similar to monocytes or myeloid dendritic cells, involving in immune responses and leukocyte activation. aLC and migLC, expressing CD83 and CCR7, represented the LC populations that were activated and began to migrate.

The authors depicted the full differentiation trajectories of LCs by analysis of HSC-LCs at multiple time-points during differentiation. SPI1, ID2, and NF-κB pathway directed LC differentiation and balanced the differentiation and activation of LCs. Pre-LCs gradually transitioned into steady-state LC1 and LC2. LC1 and LC2 branches appeared almost simultaneously during differentiation, indicating relatively independent ontogeny. EGR1 and notch signaling regulated LC1 and LC2 bifurcation.
LC1 and LC2 orchestrated reciprocal functions as revealed by analysis of the scRNA-seq data and the in vitro functional studies. LC1 had superior self-maintenance and capacity for antigen uptake, and remained stable under inflammatory microenvironment. LC2 were prone to being activated and demonstrated elevated expression of immuno-suppressive molecules (PD-L1 and RANK), and were more capable of migration and were likely to assume an immuno-regulatory role. The authors further analyzed the changes of LC subsets in psoriatic lesions. They found that the numbers of LC2 and aLC/migLC in psoriatic skin were increased, and the number of PD-L1+ T cells and CD4+Foxp3+ Treg cells in psoriatic lesions were also increased. The interaction of LCs and T cells might assume an immuno-regulatory role to counterbalance against skin inflammation.
Xiaochun Liu, a resident from Hospital for Skin Diseases, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, and Ronghui Zhu, a PhD student from Huashan Hospital, Fudan University, are the co-first authors. Xiao Liu from Tsinghua Shenzhen International Graduate School, Tsinghua University is the senior author. Prof. Wei Li is the Lead contact.
Links: https://doi.org/10.1016/j.immuni.2021.08.012


