Yifu Qiu’s team identified IRX3 as a driver of macrophage inflammatory response that promotes obesity
Source:Yifu Qiu
2021-11-17
On September 23, 2021, Dr. Yifu Qiu’s group from College of Future Technology and Center for Life Sciences at Peking University published a research article in Nature Immunology, entitled “Macrophage IRX3 promotes diet-induced obesity and metabolic inflammation”. This study reveals the mechanisms by which macrophage IRX3 promotes proinflammatory gene expression that exacerbates metabolic inflammation and associated metabolic diseases.
Obesity has been linked to numerous comorbid conditions including type 2 diabetes mellitus, nonalcoholic fatty liver disease (NAFLD), atherosclerosis, cancers as well as COVID-19 associated diseases. Previous genome-wide association studies (GWAS) discovered that the variants in the first intron of FTO are strongly associated with obesity in humans. Several groups reported that an enhancer sequence in the FTO intron directly bind to the promoter of IRX3 and promote its expression, and IRX3 plays a critical role in the regulation of body weight and insulin resistance. Global Irx3 knockout in mice reduced body weight and improved insulin sensitivity. However, the cell types in which IRX3 functions and the precise molecular mechanisms by which IRX3 regulates metabolic parameters remain unclear.
Adipose tissue macrophages play important roles in the regulation of body weight and type 2 diabetes. To investigate the role of macrophage IRX3 in the control of body weight, the researchers deleted Irx3 in myeloid lineages by crossing Irx3fl/fl with Lyz2Cre mice. In comparison to the Irx3fl/fl counterparts, Irx3fl/flLyz2Cre mice showed much lower body weight, and markedly reduced adiposity after HFD feeding. Using different thermogenesis mouse models, including cold exposure and treatment with β3 adrenergic receptor (β3 AR) agonist, they further revealed that macrophage Irx3 deficiency promotes adipocyte lipolysis and thermogenesis through modulating the β3 AR-cAMP-PKA pathway in adipocytes.
Mechanistically, the researchers demonstrated that IRX3 promotes inflammatory gene expression in macrophages via RNA-seq, Irx3 deletion and Irx3 overexpression experiments. Moreover, the luciferase reporter assays validated that IRX3 acts as a transcriptional factor to directly bind the promoter region of Il1a, Il1b and Il6. This study further uncovered that LPS activates JNK1/2 kinases to hyper-phosphorylate IRX3 at S361 and S389 sites, which is required for the activation of IRX3. Besides phosphorylation, LPS stimulation stabilizes IRX3 by inhibiting its ubiquitination at K409, which amplifies the transcriptional capacity of IRX3. Finally, using diet-induced metabolic disease mouse model, they revealed that IRX3 in adipose tissue macrophages accelerates obesity, insulin resistance and fatty liver.
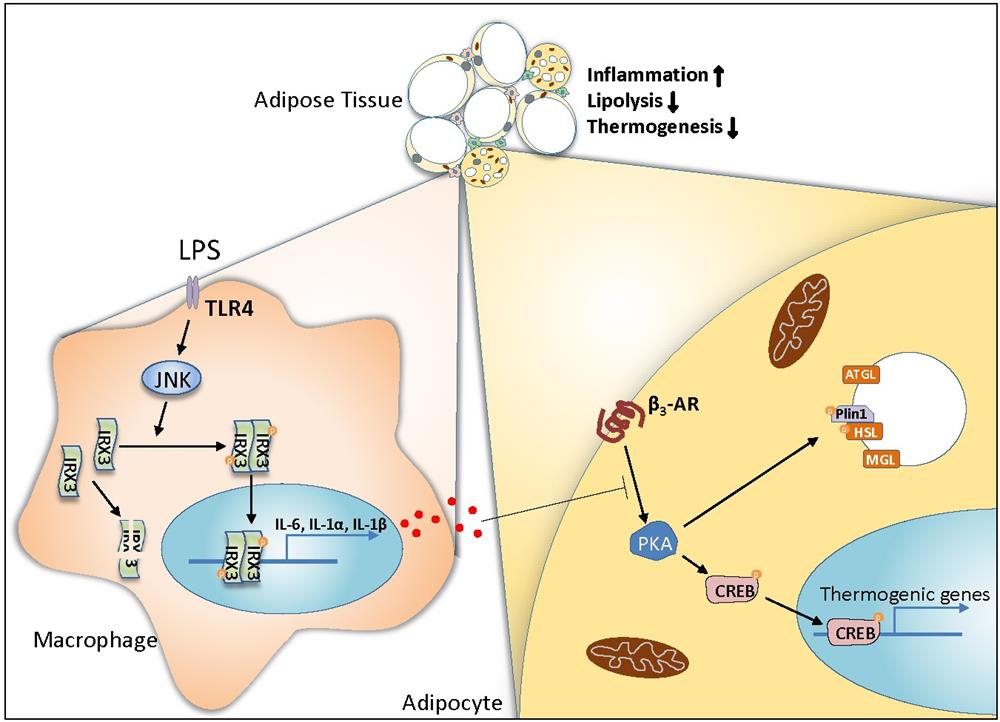
In summary, this study demonstrated that IRX3 promotes inflammatory gene expression as a transcriptional factor in macrophages, which inhibits brown and beige adipocyte lipolysis and thermogenesis and thus accelerates the development of obesity and insulin resistance. In addition, the researchers revealed the phosphorylation and de-ubiquitination events of IRX3 by LPS treatment that coordinately promotes IRX3’s function. This study implicates IRX3 as a new therapeutic target for obesity and other inflammatory-related diseases (Figure). Nature Immunology publish a news & views to highlight this study in the same issue.
Dr. Yifu Qiu from the College of Future Technology at Peking University is the corresponding author of this paper. PhD student Jingfei Yao is the first author. The work was funded in part by the National Key R&D Program of China, National Natural Science Foundation of China and the Tsinghua-Peking Center for Life Sciences.
Links: https://www.nature.com/articles/s41590-021-01023-y
Obesity has been linked to numerous comorbid conditions including type 2 diabetes mellitus, nonalcoholic fatty liver disease (NAFLD), atherosclerosis, cancers as well as COVID-19 associated diseases. Previous genome-wide association studies (GWAS) discovered that the variants in the first intron of FTO are strongly associated with obesity in humans. Several groups reported that an enhancer sequence in the FTO intron directly bind to the promoter of IRX3 and promote its expression, and IRX3 plays a critical role in the regulation of body weight and insulin resistance. Global Irx3 knockout in mice reduced body weight and improved insulin sensitivity. However, the cell types in which IRX3 functions and the precise molecular mechanisms by which IRX3 regulates metabolic parameters remain unclear.
Adipose tissue macrophages play important roles in the regulation of body weight and type 2 diabetes. To investigate the role of macrophage IRX3 in the control of body weight, the researchers deleted Irx3 in myeloid lineages by crossing Irx3fl/fl with Lyz2Cre mice. In comparison to the Irx3fl/fl counterparts, Irx3fl/flLyz2Cre mice showed much lower body weight, and markedly reduced adiposity after HFD feeding. Using different thermogenesis mouse models, including cold exposure and treatment with β3 adrenergic receptor (β3 AR) agonist, they further revealed that macrophage Irx3 deficiency promotes adipocyte lipolysis and thermogenesis through modulating the β3 AR-cAMP-PKA pathway in adipocytes.
Mechanistically, the researchers demonstrated that IRX3 promotes inflammatory gene expression in macrophages via RNA-seq, Irx3 deletion and Irx3 overexpression experiments. Moreover, the luciferase reporter assays validated that IRX3 acts as a transcriptional factor to directly bind the promoter region of Il1a, Il1b and Il6. This study further uncovered that LPS activates JNK1/2 kinases to hyper-phosphorylate IRX3 at S361 and S389 sites, which is required for the activation of IRX3. Besides phosphorylation, LPS stimulation stabilizes IRX3 by inhibiting its ubiquitination at K409, which amplifies the transcriptional capacity of IRX3. Finally, using diet-induced metabolic disease mouse model, they revealed that IRX3 in adipose tissue macrophages accelerates obesity, insulin resistance and fatty liver.

In summary, this study demonstrated that IRX3 promotes inflammatory gene expression as a transcriptional factor in macrophages, which inhibits brown and beige adipocyte lipolysis and thermogenesis and thus accelerates the development of obesity and insulin resistance. In addition, the researchers revealed the phosphorylation and de-ubiquitination events of IRX3 by LPS treatment that coordinately promotes IRX3’s function. This study implicates IRX3 as a new therapeutic target for obesity and other inflammatory-related diseases (Figure). Nature Immunology publish a news & views to highlight this study in the same issue.
Dr. Yifu Qiu from the College of Future Technology at Peking University is the corresponding author of this paper. PhD student Jingfei Yao is the first author. The work was funded in part by the National Key R&D Program of China, National Natural Science Foundation of China and the Tsinghua-Peking Center for Life Sciences.
Links: https://www.nature.com/articles/s41590-021-01023-y


