Dr. Fangfang Zhou & Long Zhang reveal a new mechanism of deacetylated-dependent IRF3/IRF7-LLPS and develop strategy to rescue innate immunosenescence
Source:Long Zhang
2022-08-02
Innate immunity plays an important role as a barrier against the invasion of exogenous pathogens. To eliminate infection and avoid harmful immune pathology, a precise and appropriate induction of type I IFNs, including IFN-β and IFN-α. In both influenza and COVID-19 pandemics, very low and delayed IFN-I induction and much higher rate of death occurred in aged patients relative to juvenile or other individuals. The previous researches indicated that innate antiviral immunity undergoes deterioration with aging; however, the underlying mechanism for age-associated degeneration of IFN signaling and related innate immunosenescence is not clear.
Recently, the team of Professor Fangfang Zhou from Soochow University, in collaboration with Professor Long Zhang’s team from Zhejiang University, published an article research entitled “Deactylation by SIRT1 enables liquid-liquid phase separation of IRF3/IRF7 in innate antiviral immunity” on Nature Immunology. This study disclosed the biological function and precise mechanism of IRF3/IRF7 LLPS in innate immune regulation and described, at the molecular level, how aging is associated with declined innate antiviral immunity. By elucidating a new deacetylation-mediated control mechanism of IRF3/IRF7 LLPS, this research revealed a previously unknown interplay between SIRT1 activity and antiviral innate immune responses. These mechanistic studies might explain the diminished innate antiviral immunity frequently found in aged patients with viral infectious diseases.
With the infection of virus, the endogenously activated IRF3 undergoes liquid-liquid phase separation, and condensates in the nucleus. DNA-binding domain (DBD) is the most critical for IRF3 to form LLPS and nuclear puncta with ISRE-DNA. Genetically encoded chemical crosslinking of proteins coupled with mass spectrometry (GECX-MS) identified the components in the IRF3 condensates in live cells. This assay identified IRF7, with multiple unique peptides, as a strong component of IRF3 condensates. IRF3 is able to prime IRF7 condensation in vivo and compartmentalizes with IRF7 to the nuclear puncta.
Further screen searching for determinant of IRF3 nuclear puncta found the deactetylase activity of SIRT1 is crucial. In cultured cells, loss-of-SIRT1 activity inhibits IFN signaling, leading to enhanced viral replication. In vivo, mice with Sirt1 conditional knockout in myeloid cell (Sirt1fl/fl Lyz2-Cre+) exhibited abolished IRF3/7 nuclear puncta formation, severely reduced production of IFN-β and IFNα, enhanced morbidity and viral load. Either site acetylation in DBD of IRF3/7, resulted from the loss-of-SIRT1 activity sufficiently shut off IRF3/7 phase separation, IFN-I promoter binding and transcription thereby promoting viral replication. In view of vital influence of the SIRT1-dependent DBD deactylation on IRF3/7 activation, reduction of SIRT1 activity prevalent in macrophages of aging population is likely to be an important cause of innate immunosenescence. In line with this analysis, reinforcing SIRT1 activity by agonists enhance IRF3/7 LLPS, IFN-I production and thus prevent innate immunosenescence in aged mice.
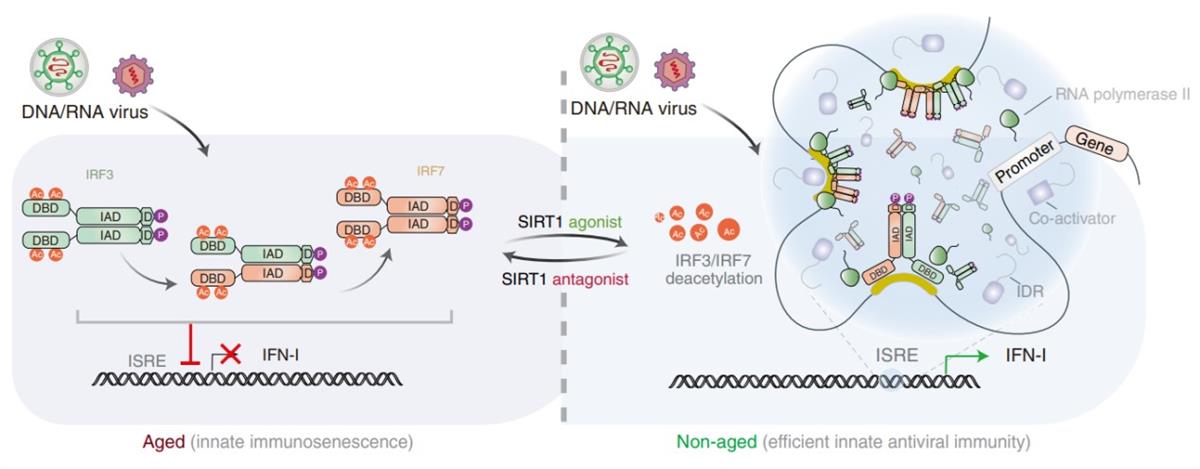
In summary, these findings not only identify a mechanism by which SIRT1 regulates IFN production by affecting IRF3/IRF7 LLPS, but also provide information on the drivers of innate immunosenescence.
Professor Fangfang Zhou ang Professor Long Zhang are the co-corresponding authors of the paper. Ph.D. candidate Ziran Qin and Ph.D. candidate Xiuwu Fang are the co-first authors of the paper. This project was funded by the Ministry of Science and Technology of China, the Chinese National Natural Science Funds, the China National Postdoctoral Program for Innovative Talents, the China Postdoctoral Science Foundation, the Zhejiang Natural Science Fund and Jiangsu National Science Foundation.
Link:https://www.nature.com/articles/s41590-022-01269-0
Research briefing:https://www.nature.com/articles/s41590-022-01270-7
Recently, the team of Professor Fangfang Zhou from Soochow University, in collaboration with Professor Long Zhang’s team from Zhejiang University, published an article research entitled “Deactylation by SIRT1 enables liquid-liquid phase separation of IRF3/IRF7 in innate antiviral immunity” on Nature Immunology. This study disclosed the biological function and precise mechanism of IRF3/IRF7 LLPS in innate immune regulation and described, at the molecular level, how aging is associated with declined innate antiviral immunity. By elucidating a new deacetylation-mediated control mechanism of IRF3/IRF7 LLPS, this research revealed a previously unknown interplay between SIRT1 activity and antiviral innate immune responses. These mechanistic studies might explain the diminished innate antiviral immunity frequently found in aged patients with viral infectious diseases.
With the infection of virus, the endogenously activated IRF3 undergoes liquid-liquid phase separation, and condensates in the nucleus. DNA-binding domain (DBD) is the most critical for IRF3 to form LLPS and nuclear puncta with ISRE-DNA. Genetically encoded chemical crosslinking of proteins coupled with mass spectrometry (GECX-MS) identified the components in the IRF3 condensates in live cells. This assay identified IRF7, with multiple unique peptides, as a strong component of IRF3 condensates. IRF3 is able to prime IRF7 condensation in vivo and compartmentalizes with IRF7 to the nuclear puncta.
Further screen searching for determinant of IRF3 nuclear puncta found the deactetylase activity of SIRT1 is crucial. In cultured cells, loss-of-SIRT1 activity inhibits IFN signaling, leading to enhanced viral replication. In vivo, mice with Sirt1 conditional knockout in myeloid cell (Sirt1fl/fl Lyz2-Cre+) exhibited abolished IRF3/7 nuclear puncta formation, severely reduced production of IFN-β and IFNα, enhanced morbidity and viral load. Either site acetylation in DBD of IRF3/7, resulted from the loss-of-SIRT1 activity sufficiently shut off IRF3/7 phase separation, IFN-I promoter binding and transcription thereby promoting viral replication. In view of vital influence of the SIRT1-dependent DBD deactylation on IRF3/7 activation, reduction of SIRT1 activity prevalent in macrophages of aging population is likely to be an important cause of innate immunosenescence. In line with this analysis, reinforcing SIRT1 activity by agonists enhance IRF3/7 LLPS, IFN-I production and thus prevent innate immunosenescence in aged mice.

In summary, these findings not only identify a mechanism by which SIRT1 regulates IFN production by affecting IRF3/IRF7 LLPS, but also provide information on the drivers of innate immunosenescence.
Professor Fangfang Zhou ang Professor Long Zhang are the co-corresponding authors of the paper. Ph.D. candidate Ziran Qin and Ph.D. candidate Xiuwu Fang are the co-first authors of the paper. This project was funded by the Ministry of Science and Technology of China, the Chinese National Natural Science Funds, the China National Postdoctoral Program for Innovative Talents, the China Postdoctoral Science Foundation, the Zhejiang Natural Science Fund and Jiangsu National Science Foundation.
Link:https://www.nature.com/articles/s41590-022-01269-0
Research briefing:https://www.nature.com/articles/s41590-022-01270-7


