Dr. Hua-Bing Li group discovered that tRNA-m1A modification served as a translational checkpoint during T cell clonal expansion
Source:Hua-Bing Li
2022-10-12
On September 22 2022, Dr. Hua-Bing LI from Shanghai Institute of Immunology Shanghai Jiao Tong University School of Medicine and his collaborators published a research paper titled “tRNA-m1A modification promotes T cell expansion via efficient MYC protein synthesis” on Nature Immunology. They report that T cells upregulate transfer RNA (tRNA) m1A58 ‘writer’ proteins TRMT61A and TRMT6, which confer m1A58 RNA modification on a specific subset of early expressed tRNAs to enhance the translation efficiency of a group of key cell cycling related proteins to promotes rapid T cell expansion after activation.
CD4+ T cells constitute the major arm of adaptive immunity. Upon antigen stimulation, naive T cells undergo rapid and profound changes to exit from the quiescent state, followed by the massive clonal expansion and differentiation that is essential for an adequate immune defense. T cells meet such massive bioenergetic and biosynthetic demands by rapidly increasing protein synthesis at the transcriptional, posttranscriptional, and translational levels. The researchers hypothesized that there might be a mechanism that T cells could be fully utilizing the existing finite pool of messenger RNAs by enhancing translation efficiency.
To probe RNA dynamics during T cell activation, they first performed RNA sequencing (RNA-seq) and tRNA sequencing at several time intervals during in vitro activation of T cells in culture. They found that different tRNA species could be classified into distinct expression clusters, and, importantly, found that tRNAs involved in translation events were most significantly altered during early T cell activation. Among many genes that were differentially expressed, we focused our interest on the m1A ‘writer’ genes TRMT61A and TRMT6, which were among the top upregulated translation-related genes during early T cell activation. Notably, both genes are known to encode proteins involved in tRNA processing, including the evolutionarily conserved epitranscriptomic mark N1-methyladenosine (m1A), although their functions in vivo, especially in the immune system, are unknown. Thus, they generated CD4+ T cell-specific conditional knockout mice and challenged these in T cell-mediated disease models, and then characterized the T cells both in vivo and in vitro.
They found that conditional deletion of TRMT61A or TRMT6 led to loss of m1A at position 58 (m1A58) on tRNAs. Moreover, loss of either gene caused cell cycle arrest and defective T cell proliferation, resulting in dysregulated T cell function and disrupted T cell homeostasis. RNA-seq analysis revealed that m1A suppression did not affect gene expression in naive T cells but did affect hundreds of genes in activated T cells. Further pathway analysis and both in vitro and in vivo rescue and codon-switch assays identified MYC translation deficiency as the main driver of proliferation defects. Lastly, we performed RiboTag RNA sequencing (RiboTag-seq) to systematically investigate m1A-mediated translation events. Published time-point proteomics data, as well as our tRNA-seq and RNA-seq data, indicate that TRMT61A-mediated tRNA-m1A58 modification is essential for the rapid upregulation of a group of tRNA species, which decode and efficiently translate a program of important pre-cell-cycling genes.
In summary, the authors demonstrate that tRNA-m1A58 methylation serves as an epigenetic translational control and constitutes an important mechanism that enables the rapid synthesis of large numbers of functional proteins and drives activated T cells to enter mitosis and proliferate quickly. Specifically, they found that: (1) translation is an active process of this genetic information processing in early T cell activation; (2) the expression of tRNAs and tRNA-m1A58 ‘writer’ proteins (TRMT6 and TRMT61A) is rapidly increased upon T cell activation in a very short time; and (3) as tRNA-m1A58 promotes translation, m1A58 in tRNA supports the rapid translation demands of activated T cells, particularly for proteins enriched with codons corresponding to tRNAs that need to be upregulated at the pre-cell-cycling stage of T cell activation. This emphasizes the importance of m1A58 modification as an important translational checkpoint to serve as a ‘gas pedal’ for T cell proliferation, in contrast to the mRNA-m6A modification that they have previously described, which rather serve to ‘release the brake’ to exit the quiescent state for T cell activation.
In the same issue of Nature Immunology, the journal highlights the work with ‘News & Views’ and ‘Research Briefing’. In the ‘Expert opinion’, the expert comments: “This manuscript is exciting as it is the first (to my knowledge) to contain mechanistic data linking tRNA modification to change in T cell function. A key strength is that it interrogates the mechanism proposed in primary T cells, showing that tRNA usage/modification can impact protein expression and cell function. This manuscript has the potential to bring novel insight to the way we think about post-transcriptional control in immune cells”. In the ‘From the editor’, the editor summarizes: “This study looks at how naive T cells upon activation can become the sports car equivalent of going from 0 to 60 mph in 2 s. They find N1-methyladenosine (m1A) modification of tRNAs by the ‘writer’ tRNA-m1A58 is a translational checkpoint that provides the ‘gas pedal’ for T cell proliferation.”
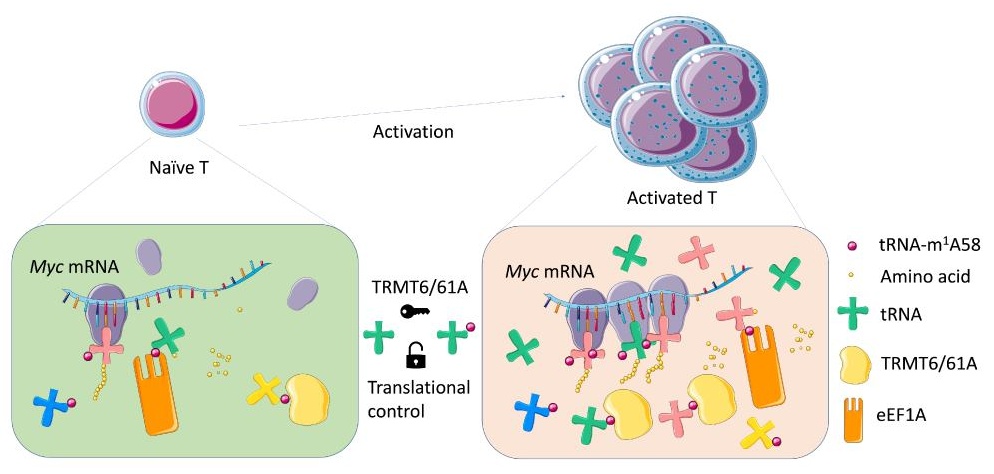 TRMT61A mediated tRNA-m1A58 modification facilitate CD4+ T cell proliferation as a translational checkpoint
TRMT61A mediated tRNA-m1A58 modification facilitate CD4+ T cell proliferation as a translational checkpoint
This study was supported by National Natural Science Foundation of China Grant, National Key R&D Program of China and Shanghai Science and Technology Commission. Drs. Yongbo Liu, Jing Zhou, Xiaoyu Li, and Mrs Jintong Shi, Xiaoting Zhang are the equally contributing first authors. Drs. Hua-Bing Li (Shanghai Jiao Tong University School of Medicine), Yuzhang Wu (Chongqing International Institute for Immunology), Chengqi yi (Peking University) and Richard Flavell (Yale University School of Medicine) are the co-corresponding authors.
Link to the paper:https://www.nature.com/articles/s41590-022-01301-3
Research Briefing: https://www.nature.com/articles/s41590-022-01302-2
News & Views: https://www.nature.com/articles/s41590-022-01317-9
CD4+ T cells constitute the major arm of adaptive immunity. Upon antigen stimulation, naive T cells undergo rapid and profound changes to exit from the quiescent state, followed by the massive clonal expansion and differentiation that is essential for an adequate immune defense. T cells meet such massive bioenergetic and biosynthetic demands by rapidly increasing protein synthesis at the transcriptional, posttranscriptional, and translational levels. The researchers hypothesized that there might be a mechanism that T cells could be fully utilizing the existing finite pool of messenger RNAs by enhancing translation efficiency.
To probe RNA dynamics during T cell activation, they first performed RNA sequencing (RNA-seq) and tRNA sequencing at several time intervals during in vitro activation of T cells in culture. They found that different tRNA species could be classified into distinct expression clusters, and, importantly, found that tRNAs involved in translation events were most significantly altered during early T cell activation. Among many genes that were differentially expressed, we focused our interest on the m1A ‘writer’ genes TRMT61A and TRMT6, which were among the top upregulated translation-related genes during early T cell activation. Notably, both genes are known to encode proteins involved in tRNA processing, including the evolutionarily conserved epitranscriptomic mark N1-methyladenosine (m1A), although their functions in vivo, especially in the immune system, are unknown. Thus, they generated CD4+ T cell-specific conditional knockout mice and challenged these in T cell-mediated disease models, and then characterized the T cells both in vivo and in vitro.
They found that conditional deletion of TRMT61A or TRMT6 led to loss of m1A at position 58 (m1A58) on tRNAs. Moreover, loss of either gene caused cell cycle arrest and defective T cell proliferation, resulting in dysregulated T cell function and disrupted T cell homeostasis. RNA-seq analysis revealed that m1A suppression did not affect gene expression in naive T cells but did affect hundreds of genes in activated T cells. Further pathway analysis and both in vitro and in vivo rescue and codon-switch assays identified MYC translation deficiency as the main driver of proliferation defects. Lastly, we performed RiboTag RNA sequencing (RiboTag-seq) to systematically investigate m1A-mediated translation events. Published time-point proteomics data, as well as our tRNA-seq and RNA-seq data, indicate that TRMT61A-mediated tRNA-m1A58 modification is essential for the rapid upregulation of a group of tRNA species, which decode and efficiently translate a program of important pre-cell-cycling genes.
In summary, the authors demonstrate that tRNA-m1A58 methylation serves as an epigenetic translational control and constitutes an important mechanism that enables the rapid synthesis of large numbers of functional proteins and drives activated T cells to enter mitosis and proliferate quickly. Specifically, they found that: (1) translation is an active process of this genetic information processing in early T cell activation; (2) the expression of tRNAs and tRNA-m1A58 ‘writer’ proteins (TRMT6 and TRMT61A) is rapidly increased upon T cell activation in a very short time; and (3) as tRNA-m1A58 promotes translation, m1A58 in tRNA supports the rapid translation demands of activated T cells, particularly for proteins enriched with codons corresponding to tRNAs that need to be upregulated at the pre-cell-cycling stage of T cell activation. This emphasizes the importance of m1A58 modification as an important translational checkpoint to serve as a ‘gas pedal’ for T cell proliferation, in contrast to the mRNA-m6A modification that they have previously described, which rather serve to ‘release the brake’ to exit the quiescent state for T cell activation.
In the same issue of Nature Immunology, the journal highlights the work with ‘News & Views’ and ‘Research Briefing’. In the ‘Expert opinion’, the expert comments: “This manuscript is exciting as it is the first (to my knowledge) to contain mechanistic data linking tRNA modification to change in T cell function. A key strength is that it interrogates the mechanism proposed in primary T cells, showing that tRNA usage/modification can impact protein expression and cell function. This manuscript has the potential to bring novel insight to the way we think about post-transcriptional control in immune cells”. In the ‘From the editor’, the editor summarizes: “This study looks at how naive T cells upon activation can become the sports car equivalent of going from 0 to 60 mph in 2 s. They find N1-methyladenosine (m1A) modification of tRNAs by the ‘writer’ tRNA-m1A58 is a translational checkpoint that provides the ‘gas pedal’ for T cell proliferation.”

This study was supported by National Natural Science Foundation of China Grant, National Key R&D Program of China and Shanghai Science and Technology Commission. Drs. Yongbo Liu, Jing Zhou, Xiaoyu Li, and Mrs Jintong Shi, Xiaoting Zhang are the equally contributing first authors. Drs. Hua-Bing Li (Shanghai Jiao Tong University School of Medicine), Yuzhang Wu (Chongqing International Institute for Immunology), Chengqi yi (Peking University) and Richard Flavell (Yale University School of Medicine) are the co-corresponding authors.
Link to the paper:https://www.nature.com/articles/s41590-022-01301-3
Research Briefing: https://www.nature.com/articles/s41590-022-01302-2
News & Views: https://www.nature.com/articles/s41590-022-01317-9


