Zhiwei Huang’s group revealed the molecular mechanism of human B-cell receptor in a paper published in Science
Source:Zhiwei Huang
2022-09-06
On August 18th, a paper titled "Cryo-EM structures of two human B cell receptor isotypes " was published in Science by the Zhiwei Huang group from in Harbin Institute of Technology, revealed the assembling and recognition mechanism of BCR complex subunits, and found the assembling pattern of different subtypes of BCR was conserved in the membrane, but different in the exocytosis.
Human adaptive immune cells (T cells and B cells) play a key role in pathogenic infections, carcinogenesis, and autoimmune diseases. T and B cells recognize antigenic signals through T cell receptor (TCR) and B cell receptor (BCR), respectively, and transmit the signals across the membrane into the cell to activate the immune response of T and B cells. T and B cell receptors belong to a class of the most complex cell receptors composed of multiple proteins, which play a crucial role in the development, differentiation and function of T and B cells. The complex signal transduction, the structural basis and molecular mechanism of immune activation of TCR and BCR have always been an important basic scientific problem in immunology.
There are five subtypes of human B-cell receptors. In this study, the group analysed the structure of two BCR complexes which are human IgG and IgM. The BCR complex contains a membrane-bound form of immunoglobulin (mIg) homodimer, which is used to recognize antigens, and a membrane-bound form of Igα/β (CD79α/CD79β) heterodimer for signaling (stoichiometric ratio 1:1). Among them, the mIg dimer contains Fab and Fc domains, connecting peptide (CPs) and TM helices, and the Igα/β structure consists of two extracellular Ig-like domains, CPs and TM helices. The assembly of BCR complex is carried out by extracellular mIg homodimer (IgG-Cγ3 and IgM-Cμ4, respectively), Ig-like domains of Igα/β, connecting peptides and transmembrane helices. Through structural comparison of the two isoforms, the group found that the transmembrane helical regions of mIgG and mIgM bind to Igα/β through conserved hydrophobic and polar interactions. In contrast with the extracellular assembly of Ig-like domains of Igα/β with IgG-Cγ3 through the head-to-tail mode, IgM-Cµ4 forms side-to-side contacts with the Ig-like domains of Igα/β. And the CD loop of Igα swings about 90 degrees to contact IgG-Cγ3 and IgM-Cμ4. Whether the structurally observed different isoform assembly patterns are related to activity warrants further investigation.
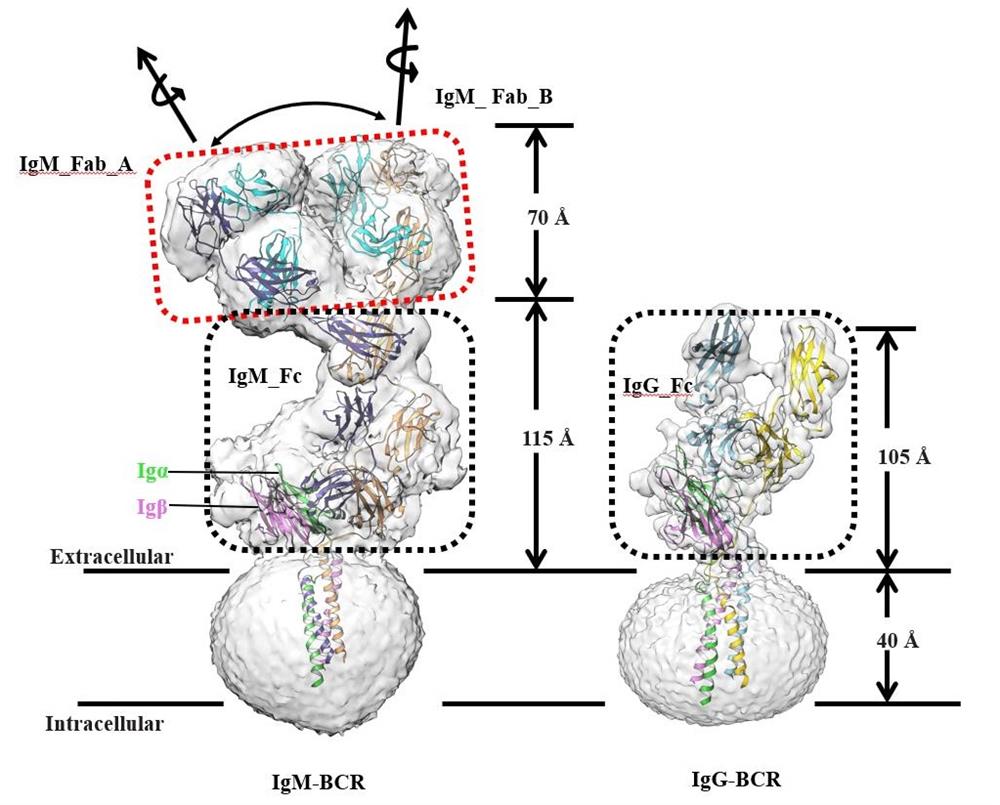
Secretory IgM usually forms pentamers, but only the monomeric state of IgM is observed on the membrane-bound BCR in resting state. The Ig-like domain of Igα and membrane-bound IgM-Cμ4 completely coincide, which explains the monomeric state of membrane-bound IgM-BCR. The activation of BCR is usually accompanied by the BCR clustering. In the resting state, the Ig-like domain of Igα/β binds to Cμ4 or Cγ3, which sterically blocks mIg oligomerization, while the binding of antigen may exert mechanical force on the Fab domain to trigger structural changes of mIg_Fc, thereby releasing the clustering interface of Cγ3 or Cµ4 shaded by Igα/β, leading to IgM-BCR clustering, and signaling. The underlying mechanism needs to be further investigated. Electron densitometric analysis clearly identified 6 and 14 glycosylation sites on IgG and IgM-BCR, respectively.
These data above not only resolve the long-standing mystery about the structure and assembly mechanism of BCR, but also provide a key structural basis for understanding the molecular mechanism of BCR initiating immune response and developing immunotherapies targeting BCR for the treatment of related diseases.
A contemporary-published commentary article "Unveiling the B cell receptor structure - Molecular structures provide a road map for understanding and controlling B cell receptor activation" in Perspective Section on Science introduced the research results.
In recent years, in the study of the structure and molecular mechanism of human immune cell receptors, the Zhiwei Huang group first resolved the three-dimensional structure of human TCR complex by solving technical problems such as the dynamic complexity of TCR and BCR complex, and revealed the subunit assembly and recognition mechanism of TCR complex (Nature, 2019). Through further analysis of high-resolution TCR complex structure, the group found that there was a "cholesterol binding channel" in the transmembrane region of TCR (Molecular Cell, 2022). Cholesterol molecules bind to this channel to inhibit TCR activation, and the structural basis of TCR activation is revealed by removing cholesterol molecules to cause TCR constitutive activation. Thus, the "cholesterol - deadbolt" control theory of TCR is proposed, which provides a theoretical basis for rational design of immunotherapy targeting TCR to regulate T cell activity.
Prof. Zhiwei Huang from Harbin Institute of Technology is the corresponding author of this paper. Mr. Xinyu Ma (PhD), Mr. Yuwei Zhu (Associate Research Fellow), Mr. De Dong (Postdoctor) and Ms. Yan Chen (Postdoctor) are the co-first authors of this paper. Mr. Shubo Wang (PhD), Ms. Fan Zhang (Research Fellow) and Mr. Changyou Guo (PhD) participated in part of the research. This project is supported by National Natural Science Foundation of China, Tencent Science Foundation and HIT Young Scientist Studio.
The paper links: http://doi.org/10.1126/science.abo3828
Human adaptive immune cells (T cells and B cells) play a key role in pathogenic infections, carcinogenesis, and autoimmune diseases. T and B cells recognize antigenic signals through T cell receptor (TCR) and B cell receptor (BCR), respectively, and transmit the signals across the membrane into the cell to activate the immune response of T and B cells. T and B cell receptors belong to a class of the most complex cell receptors composed of multiple proteins, which play a crucial role in the development, differentiation and function of T and B cells. The complex signal transduction, the structural basis and molecular mechanism of immune activation of TCR and BCR have always been an important basic scientific problem in immunology.
There are five subtypes of human B-cell receptors. In this study, the group analysed the structure of two BCR complexes which are human IgG and IgM. The BCR complex contains a membrane-bound form of immunoglobulin (mIg) homodimer, which is used to recognize antigens, and a membrane-bound form of Igα/β (CD79α/CD79β) heterodimer for signaling (stoichiometric ratio 1:1). Among them, the mIg dimer contains Fab and Fc domains, connecting peptide (CPs) and TM helices, and the Igα/β structure consists of two extracellular Ig-like domains, CPs and TM helices. The assembly of BCR complex is carried out by extracellular mIg homodimer (IgG-Cγ3 and IgM-Cμ4, respectively), Ig-like domains of Igα/β, connecting peptides and transmembrane helices. Through structural comparison of the two isoforms, the group found that the transmembrane helical regions of mIgG and mIgM bind to Igα/β through conserved hydrophobic and polar interactions. In contrast with the extracellular assembly of Ig-like domains of Igα/β with IgG-Cγ3 through the head-to-tail mode, IgM-Cµ4 forms side-to-side contacts with the Ig-like domains of Igα/β. And the CD loop of Igα swings about 90 degrees to contact IgG-Cγ3 and IgM-Cμ4. Whether the structurally observed different isoform assembly patterns are related to activity warrants further investigation.

Secretory IgM usually forms pentamers, but only the monomeric state of IgM is observed on the membrane-bound BCR in resting state. The Ig-like domain of Igα and membrane-bound IgM-Cμ4 completely coincide, which explains the monomeric state of membrane-bound IgM-BCR. The activation of BCR is usually accompanied by the BCR clustering. In the resting state, the Ig-like domain of Igα/β binds to Cμ4 or Cγ3, which sterically blocks mIg oligomerization, while the binding of antigen may exert mechanical force on the Fab domain to trigger structural changes of mIg_Fc, thereby releasing the clustering interface of Cγ3 or Cµ4 shaded by Igα/β, leading to IgM-BCR clustering, and signaling. The underlying mechanism needs to be further investigated. Electron densitometric analysis clearly identified 6 and 14 glycosylation sites on IgG and IgM-BCR, respectively.
These data above not only resolve the long-standing mystery about the structure and assembly mechanism of BCR, but also provide a key structural basis for understanding the molecular mechanism of BCR initiating immune response and developing immunotherapies targeting BCR for the treatment of related diseases.
A contemporary-published commentary article "Unveiling the B cell receptor structure - Molecular structures provide a road map for understanding and controlling B cell receptor activation" in Perspective Section on Science introduced the research results.
In recent years, in the study of the structure and molecular mechanism of human immune cell receptors, the Zhiwei Huang group first resolved the three-dimensional structure of human TCR complex by solving technical problems such as the dynamic complexity of TCR and BCR complex, and revealed the subunit assembly and recognition mechanism of TCR complex (Nature, 2019). Through further analysis of high-resolution TCR complex structure, the group found that there was a "cholesterol binding channel" in the transmembrane region of TCR (Molecular Cell, 2022). Cholesterol molecules bind to this channel to inhibit TCR activation, and the structural basis of TCR activation is revealed by removing cholesterol molecules to cause TCR constitutive activation. Thus, the "cholesterol - deadbolt" control theory of TCR is proposed, which provides a theoretical basis for rational design of immunotherapy targeting TCR to regulate T cell activity.
Prof. Zhiwei Huang from Harbin Institute of Technology is the corresponding author of this paper. Mr. Xinyu Ma (PhD), Mr. Yuwei Zhu (Associate Research Fellow), Mr. De Dong (Postdoctor) and Ms. Yan Chen (Postdoctor) are the co-first authors of this paper. Mr. Shubo Wang (PhD), Ms. Fan Zhang (Research Fellow) and Mr. Changyou Guo (PhD) participated in part of the research. This project is supported by National Natural Science Foundation of China, Tencent Science Foundation and HIT Young Scientist Studio.
The paper links: http://doi.org/10.1126/science.abo3828


