Prof. Xin Lin’s group revealed the role of CARD9 mutation in regulating allergic bronchopulmonary aspergillosis (ABPA) pathogenesis
Source:Xin Lin
2018-06-11
On May 18th, Prof. Xin Lin’s group from Tsinghua University together with Prof. Xinming Jia’s group from Tongji University published a paper named “CARD9S12N mutation converts alveolar macrophages into IL-5-producing cells and facilitates type 2 immune responses” in Nature Immunology. This paper reported that a single-nucleotide polymorphism of Card9, resulting in the substitution S12N (CARD9S12N), would turn it into a susceptibility gene in allergic bronchopulmonary aspergillosis (ABPA) and further illustrated the underlying molecular mechanism in ABPA pathogenesis.
The pathogenic microorganism responsible for ABPA is Aspergillus fumigatus. At the estimation that a person on average inhales hundreds of A. fumigatus conidia every day, normal innate immune system can generate rapid and effective response in immunocompetent hosts that protects them from invasive infection, while A. fumigatus conidia inhaled by immunocompromised hosts will stick to the airway tissue with excessive mucus and induce severe allergic reactions. ABPA displays a 1 to 12.9% rate in chronic bronchial asthma patients and a 7 to 9% rate in cystic fibrosis patients, while this prevalence rate of ABPA can reach up to 38.6% among patients with severe bronchial asthma admitted to the ICU. With the consideration that ABPA might be a significant risk factor involved in the death of asthma patients, the mechanism of ABPA pathogenesis still remains unknown.
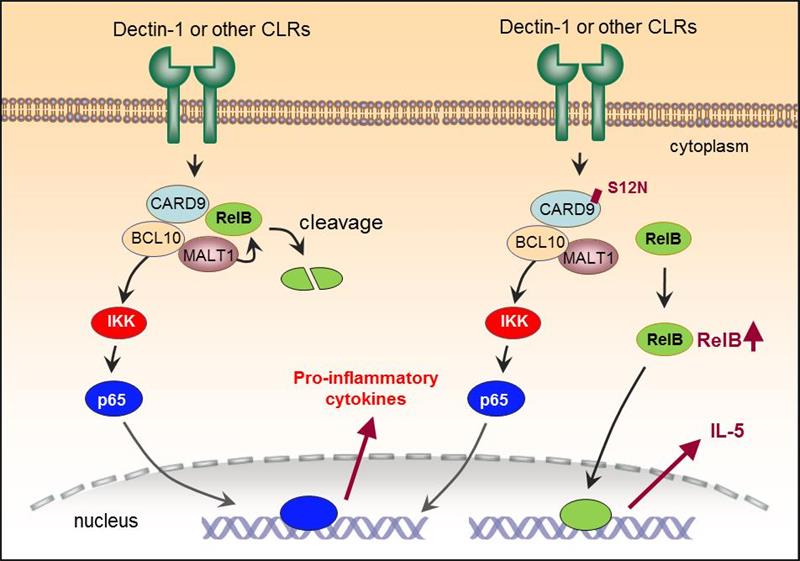
CARD9 is a key adaptor protein downstream of C-type lectin receptor (CLR) signaling. Under normal conditions, CARD9 can mediate canonical NF-κB (p65) signaling pathway, which leads to the differentiation and activation of Th17 and Th1 cells in adaptive immunity to fight against fungal infection. In cooperation with Prof. Jinfu Xu from Shanghai Pulmonary Hospital, Prof. Xin Lin’s group as well as Prof. Xinming Jia’s group conducted Card9 genome screening in ABPA patients and found that Card9 gene in PBMCs from ABPA patients harbored high frequency S12N mutation. By generating the CARD9S12N knock-in mice, they found that the mice displayed a Th2 cell-mediated allergic response after A. fumigatus infection, which was highly similar to that in human ABPA patients. Through further investigation in CARD9S12N knock-in mice they discovered that A. fumigatus could activate noncanonical NF-κB (RelB) signaling pathway after recognized by C-type lectin receptor, and eosinophils would be activated through IL-5 production to promote Th2 cell differentiation as well as allergic response. Simultaneously, they verified that in PBMCs from patients with CARD9S12N polymorphism, A. fumigatus stimulation would promote the production of IL-5, which was resulted from RelB activation. This research systemically demonstrated the pathogenesis of ABPA caused by CARD9S12N polymorphism, thus would shed light on developing new therapeutic targets and strategies in curing ABPA.
Prof. Xin Lin’s group has been working on the mechanisms of CARD family proteins-mediated NF-κB activation and their impact on immune cell functions for many years, while Prof. Xinming Jia’s group has focused their work on immune responses induced by fungal infection. During last several years, the collaboration between these two groups had achieved a series of research findings in the field of anti-fungal immunity, with the related results published in several high-profile journals of immunology and medical science, including Nature Immunology, Nature Medicine, Immunity, The Journal of Experimental Medicine, etc. This research revealed the role of CARD9S12N polymorphism in activating noncanonical NF-κB pathway provides new mechanistic bases for demonstrating the function of innate immune cells in mediating allergic responses.
Full Text Link: https://www.nature.com/articles/s41590-018-0112-4
The pathogenic microorganism responsible for ABPA is Aspergillus fumigatus. At the estimation that a person on average inhales hundreds of A. fumigatus conidia every day, normal innate immune system can generate rapid and effective response in immunocompetent hosts that protects them from invasive infection, while A. fumigatus conidia inhaled by immunocompromised hosts will stick to the airway tissue with excessive mucus and induce severe allergic reactions. ABPA displays a 1 to 12.9% rate in chronic bronchial asthma patients and a 7 to 9% rate in cystic fibrosis patients, while this prevalence rate of ABPA can reach up to 38.6% among patients with severe bronchial asthma admitted to the ICU. With the consideration that ABPA might be a significant risk factor involved in the death of asthma patients, the mechanism of ABPA pathogenesis still remains unknown.
CARD9 is a key adaptor protein downstream of C-type lectin receptor (CLR) signaling. Under normal conditions, CARD9 can mediate canonical NF-κB (p65) signaling pathway, which leads to the differentiation and activation of Th17 and Th1 cells in adaptive immunity to fight against fungal infection. In cooperation with Prof. Jinfu Xu from Shanghai Pulmonary Hospital, Prof. Xin Lin’s group as well as Prof. Xinming Jia’s group conducted Card9 genome screening in ABPA patients and found that Card9 gene in PBMCs from ABPA patients harbored high frequency S12N mutation. By generating the CARD9S12N knock-in mice, they found that the mice displayed a Th2 cell-mediated allergic response after A. fumigatus infection, which was highly similar to that in human ABPA patients. Through further investigation in CARD9S12N knock-in mice they discovered that A. fumigatus could activate noncanonical NF-κB (RelB) signaling pathway after recognized by C-type lectin receptor, and eosinophils would be activated through IL-5 production to promote Th2 cell differentiation as well as allergic response. Simultaneously, they verified that in PBMCs from patients with CARD9S12N polymorphism, A. fumigatus stimulation would promote the production of IL-5, which was resulted from RelB activation. This research systemically demonstrated the pathogenesis of ABPA caused by CARD9S12N polymorphism, thus would shed light on developing new therapeutic targets and strategies in curing ABPA.

Prof. Xin Lin’s group has been working on the mechanisms of CARD family proteins-mediated NF-κB activation and their impact on immune cell functions for many years, while Prof. Xinming Jia’s group has focused their work on immune responses induced by fungal infection. During last several years, the collaboration between these two groups had achieved a series of research findings in the field of anti-fungal immunity, with the related results published in several high-profile journals of immunology and medical science, including Nature Immunology, Nature Medicine, Immunity, The Journal of Experimental Medicine, etc. This research revealed the role of CARD9S12N polymorphism in activating noncanonical NF-κB pathway provides new mechanistic bases for demonstrating the function of innate immune cells in mediating allergic responses.
Full Text Link: https://www.nature.com/articles/s41590-018-0112-4


