Researchers in China depicted the pan-cancer single-cell landscape of tumor-infiltrating T cells
Source:Zemin Zhang
2022-01-04
The team led by Professor Zemin Zhang from Biomedical Pioneering Innovation Center (BIOPIC), Beijing Advanced Innovation Center for Genomics (ICG), School of Life Sciences in Peking University, in collaboration with Jiafu Ji’s and Zhaode Bu’s teams from Peking University Cancer Hospital, and teams from Peking University Third Hospital, published a research article entitled “Pan-Cancer Single-Cell Landscape of Tumor-Infiltrating T Cells” on Science on Dec. 17th, 2021. The researchers combined gene expression profiles and T cell receptor sequences, investigated the heterogeneity and dynamics of tumor-infiltrating T cells, and performed a systematic comparison of T cells among cancer types.
T cells play a central role in eliminating tumors. Cytotoxic T cells are the major subtype that could directly kill cancer cells. However, during the process of the tumorigenesis and progression, tumor-infiltrating T cells usually differentiate into the dysfunctional state, which is widely known as T cell exhaustion. In addiiton, regulatory T cells (Treg) inhibit the functions of cytotoxic T cells, thus limiting the autologous tumor-killing activity. Better understanding the process and regulation of T cell exhaustion and Treg differentiation will help modulate the tumor-infiltrating T cells for tumor immunotherapy.
Immunotherapies that target tumor-infiltrating T cells, like immune checkpoint blockade (ICB), have shown tremendous clinical success. Single-cell RNA sequencing (scRNA-seq) has been successfully applied to the analyses of the tumor microenvironments (TMEs) among a variety of cancers, thereby contributing to the characterization of TME and novel drug targets discovery. However, cancer exhibit high heterogeneity, including the differences in the composition and states of tumor-infiltrating T cells, which may impact the variation of immunotherapeutic efficacy. There was still a lack of systematic comparison of tumor-infiltrating T cells across cancer types.
Zhang’s team and collaborators had previously performed T cell studies by scRNA-seq on three individual cancer types, including liver cancer, lung cancer, and colorectal cancer. To better depict the landscape of tumor-infiltrating T cells and reveal the commonalities and differences of T cell states in different tumor microenvironments, the researchers went on collecting single cell sequencing T cells for more cancer types, including myeloma, lymphoma, kidney cancer, ovarian cancer, endometrial cancer, esophageal cancer, thyroid cancer, breast cancer, gastric cancer, and pancreatic cancer, and also collected published data. The authors utilized novel bioinformatics methods to correct for confounding factors and batch effects, and integrated datasets from different platforms and sources. A high-resolution pan-cancer T cell atlas that contains 397,810 high-quality T cells from 316 patients across 21 cancer types was thereby constructed.
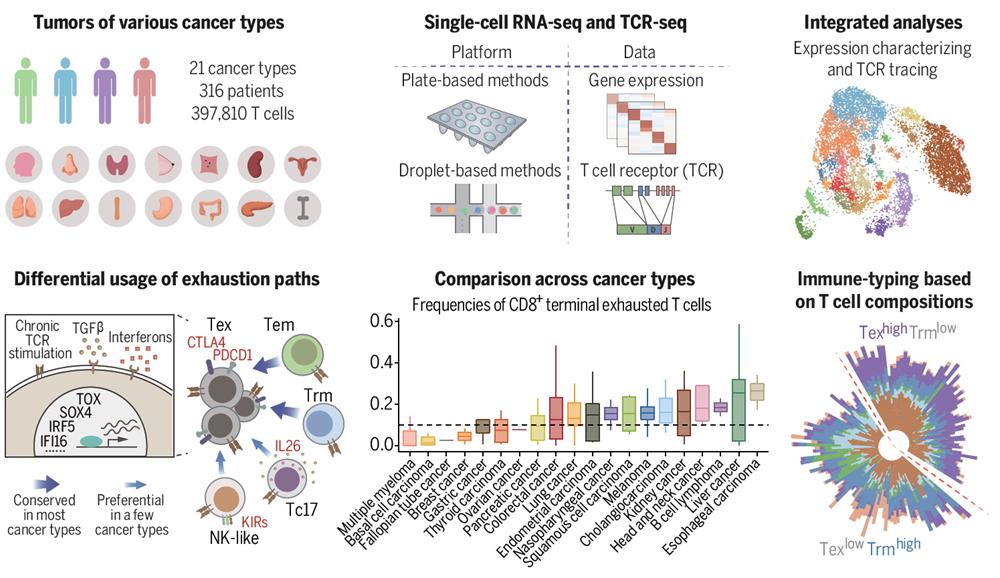
The authors identified 17 CD8+ and 24 CD4+ T cell meta-clusters with distinct features. T cells from tumors are different in composition with those from peripheral blood and para-carcinoma tissues. Specifically, CD8+ T cells in the tumor were featured by the emergence of exhausted T cells, whereas among CD4+ T cell populations, the most abundant population was the TNFRSF9+ Treg cells. The results indicate that the TMEs have reshaped the states of T cells.
In the CD8+ compartment, the exhausted T cells were found to be the major potentially tumor-reactive T cells (pTRTs). The researchers identified two main developmental paths to T cell exhaustion, through effector memory T cells and tissue-resident memory T cells, respectively, both of which were prevalent among cancer types. In addition, KIR+ NK-like T cells, Tc17 cells, or CD8+FOXP3+ cells could also differentiate to exhausted T cells, but these transitions showed certain cancer-type preference. The researchers further identified the transcription factors (TFs) associated with T cell exhaustion. TFs such as TOX and PRDM1 were highly expressed in exhausted T cells in most cancer types, but certain TFs showed cancer-type preference, such as SOX4 and FOXP3. Furthermore, the researchers proposed that interferons and TGF-β in the tumor microenvironment also influenced the exhaustion of T cells in addition to persistent TCR signaling. These results advanced the understanding of T cell exhaustion.
In the CD4+ compartment, the major potentially tumor-reactive T cells were IFNG+ TFH/TH1 and TNFRSF9+ Treg cells. The authors inferred that IFNG+ TFH/TH1 cells may be differentiated from classic IL21+ TFH cells. TNFRSF9+ Treg cells were mainly derived from resting TNFRSF9- Treg cells, but also exhibited certain state transition potentials with non-Treg cells such as TH17 and TFH in a few cancer types. This research also proposed several previously unnoticed signature genes of TNFRSF9+ Treg cells, such as the transcription factor HIVEP1, which may regulate the transcription of other signature genes including TNFRSF4, TNFRSF9 and ID3.
The authors also examined various elements associated with T cell compositions in the tumor. For example, the tumor mutation burden (TMB) showed a positive association with TFH/TH1 cells. FAT1 mutations exhibited a positive correlation with TNFRSF9+ Treg cells. Cancer types exerted an extensive impact on the frequencies of T cell populations. Moreover, the authors proposed to stratify patients based on the composition of tumor-infiltrating T cells alone. The patients could be divided into two groups — one with a higher proportion of terminally exhausted CD8+ T cells (TexhiTrmlo) and the other one with a higher proportion of tissue-resident memory CD8+ T cells (TexloTrmhi). The latter group exhibited better survival than the former. These findings will help understand the overall tumor-infiltrating T cell properties and promote the development of new strategies targeting T cells to treat human cancer.
In summary, the authors investigated the heterogeneity, dynamics, and transcriptional regulation of tumor-infiltrating T cells. This study would deepen the understanding of tumor immunology and facilitate the drug development in treating cancer.
Dr. Liangtao Zheng, Ph.D. candidate Shishang Qin, and Dr. Wen Si are the co-first authors of the paper. Drs. Zemin Zhang, Jiafu Ji, Zhaode Bu, and Dr. Xueda Hu (from Analytical Biosciences) are the co-corresponding authors of the paper. The project was funded by the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Beijing Advanced Innovation Centre for Genomics at Peking University, and supported by the Computing Platform of the Center for Life Science, Peking University.
Links: https://www.science.org/doi/10.1126/science.abe6474
T cells play a central role in eliminating tumors. Cytotoxic T cells are the major subtype that could directly kill cancer cells. However, during the process of the tumorigenesis and progression, tumor-infiltrating T cells usually differentiate into the dysfunctional state, which is widely known as T cell exhaustion. In addiiton, regulatory T cells (Treg) inhibit the functions of cytotoxic T cells, thus limiting the autologous tumor-killing activity. Better understanding the process and regulation of T cell exhaustion and Treg differentiation will help modulate the tumor-infiltrating T cells for tumor immunotherapy.
Immunotherapies that target tumor-infiltrating T cells, like immune checkpoint blockade (ICB), have shown tremendous clinical success. Single-cell RNA sequencing (scRNA-seq) has been successfully applied to the analyses of the tumor microenvironments (TMEs) among a variety of cancers, thereby contributing to the characterization of TME and novel drug targets discovery. However, cancer exhibit high heterogeneity, including the differences in the composition and states of tumor-infiltrating T cells, which may impact the variation of immunotherapeutic efficacy. There was still a lack of systematic comparison of tumor-infiltrating T cells across cancer types.
Zhang’s team and collaborators had previously performed T cell studies by scRNA-seq on three individual cancer types, including liver cancer, lung cancer, and colorectal cancer. To better depict the landscape of tumor-infiltrating T cells and reveal the commonalities and differences of T cell states in different tumor microenvironments, the researchers went on collecting single cell sequencing T cells for more cancer types, including myeloma, lymphoma, kidney cancer, ovarian cancer, endometrial cancer, esophageal cancer, thyroid cancer, breast cancer, gastric cancer, and pancreatic cancer, and also collected published data. The authors utilized novel bioinformatics methods to correct for confounding factors and batch effects, and integrated datasets from different platforms and sources. A high-resolution pan-cancer T cell atlas that contains 397,810 high-quality T cells from 316 patients across 21 cancer types was thereby constructed.

The authors identified 17 CD8+ and 24 CD4+ T cell meta-clusters with distinct features. T cells from tumors are different in composition with those from peripheral blood and para-carcinoma tissues. Specifically, CD8+ T cells in the tumor were featured by the emergence of exhausted T cells, whereas among CD4+ T cell populations, the most abundant population was the TNFRSF9+ Treg cells. The results indicate that the TMEs have reshaped the states of T cells.
In the CD8+ compartment, the exhausted T cells were found to be the major potentially tumor-reactive T cells (pTRTs). The researchers identified two main developmental paths to T cell exhaustion, through effector memory T cells and tissue-resident memory T cells, respectively, both of which were prevalent among cancer types. In addition, KIR+ NK-like T cells, Tc17 cells, or CD8+FOXP3+ cells could also differentiate to exhausted T cells, but these transitions showed certain cancer-type preference. The researchers further identified the transcription factors (TFs) associated with T cell exhaustion. TFs such as TOX and PRDM1 were highly expressed in exhausted T cells in most cancer types, but certain TFs showed cancer-type preference, such as SOX4 and FOXP3. Furthermore, the researchers proposed that interferons and TGF-β in the tumor microenvironment also influenced the exhaustion of T cells in addition to persistent TCR signaling. These results advanced the understanding of T cell exhaustion.
In the CD4+ compartment, the major potentially tumor-reactive T cells were IFNG+ TFH/TH1 and TNFRSF9+ Treg cells. The authors inferred that IFNG+ TFH/TH1 cells may be differentiated from classic IL21+ TFH cells. TNFRSF9+ Treg cells were mainly derived from resting TNFRSF9- Treg cells, but also exhibited certain state transition potentials with non-Treg cells such as TH17 and TFH in a few cancer types. This research also proposed several previously unnoticed signature genes of TNFRSF9+ Treg cells, such as the transcription factor HIVEP1, which may regulate the transcription of other signature genes including TNFRSF4, TNFRSF9 and ID3.
The authors also examined various elements associated with T cell compositions in the tumor. For example, the tumor mutation burden (TMB) showed a positive association with TFH/TH1 cells. FAT1 mutations exhibited a positive correlation with TNFRSF9+ Treg cells. Cancer types exerted an extensive impact on the frequencies of T cell populations. Moreover, the authors proposed to stratify patients based on the composition of tumor-infiltrating T cells alone. The patients could be divided into two groups — one with a higher proportion of terminally exhausted CD8+ T cells (TexhiTrmlo) and the other one with a higher proportion of tissue-resident memory CD8+ T cells (TexloTrmhi). The latter group exhibited better survival than the former. These findings will help understand the overall tumor-infiltrating T cell properties and promote the development of new strategies targeting T cells to treat human cancer.
In summary, the authors investigated the heterogeneity, dynamics, and transcriptional regulation of tumor-infiltrating T cells. This study would deepen the understanding of tumor immunology and facilitate the drug development in treating cancer.
Dr. Liangtao Zheng, Ph.D. candidate Shishang Qin, and Dr. Wen Si are the co-first authors of the paper. Drs. Zemin Zhang, Jiafu Ji, Zhaode Bu, and Dr. Xueda Hu (from Analytical Biosciences) are the co-corresponding authors of the paper. The project was funded by the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Beijing Advanced Innovation Centre for Genomics at Peking University, and supported by the Computing Platform of the Center for Life Science, Peking University.
Links: https://www.science.org/doi/10.1126/science.abe6474


