Huang Bo's team reveals a new mechanism of SARS-COV-2 Delta mutant strains utilizing macrophage endosomal pH amplification and targeted therapy strategies
Source:Yabo Zhou
2022-01-10
On 17 December, 2021, the team of Professor Huang Bo from the Institute of Basic Medicine of the Chinese Academy of Medical Sciences published two study titled "Escaping alveolar macrophage endosomal retention explains massive expansion of SARS-CoV-2 Delta Variant" in Signal Transduction and Targeted Therapy, and titled " SARS-CoV-2 treatment effects induced by ACE2-expressing microparticles are explained by the oxidized cholesterol-increased endosomal pH of alveolar macrophages" in Cellular & Molecular Immunology, it was discovered for the first time that the delta mutant strain can utilize its spike protein to protonate, thereby escaping from the endocytic corpuscles, and develop a new targeted therapy strategies: AO-MPs, as a natural biomaterial, can function as an accelerator of viral clearance, leading to the treatment of SARS-CoV-2 infection with high efficacy and safety.
The SARS-COV-2 has caused a large number of infections since it was discovered at the end of 2019. As the increase in the number of infections and the continuation of the epidemic, SARS-CoV-2 continues to evolve and mutate, resulting in numerous virus variants, some of which have significantly improved transmission capacity and pathogenicity. At the meantime, there are also immune escape phenomena which were found in some strains, then these mutant strains have aroused the attention of public health departments and the public. Presently, the SARS-CoV-2 Delta variant (B.1.617.2) has significant replication and dissemination capabilities, and it is dominant in the world. It is very harmful to humans, but how the Delta variants with T478K, P681R and L452R mutations achieve their ultra-fast transmission is still unknown.
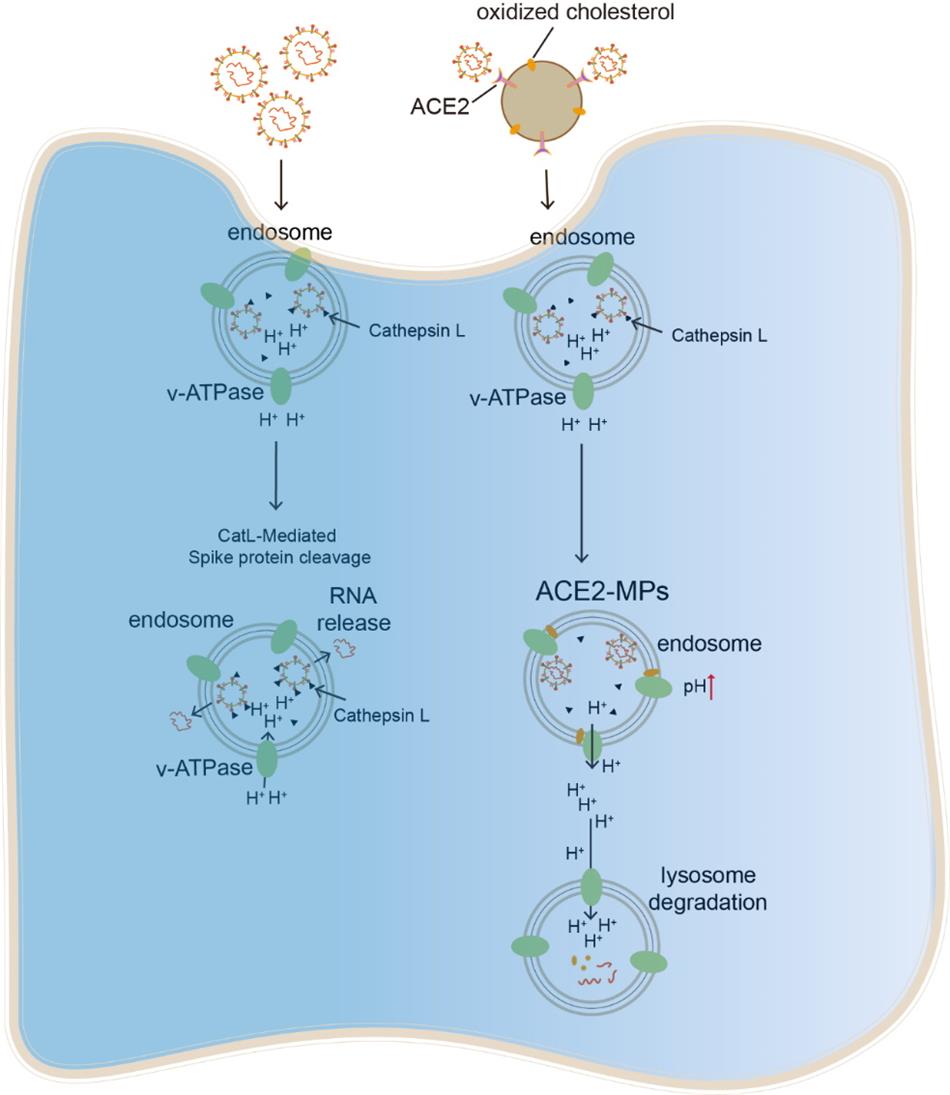
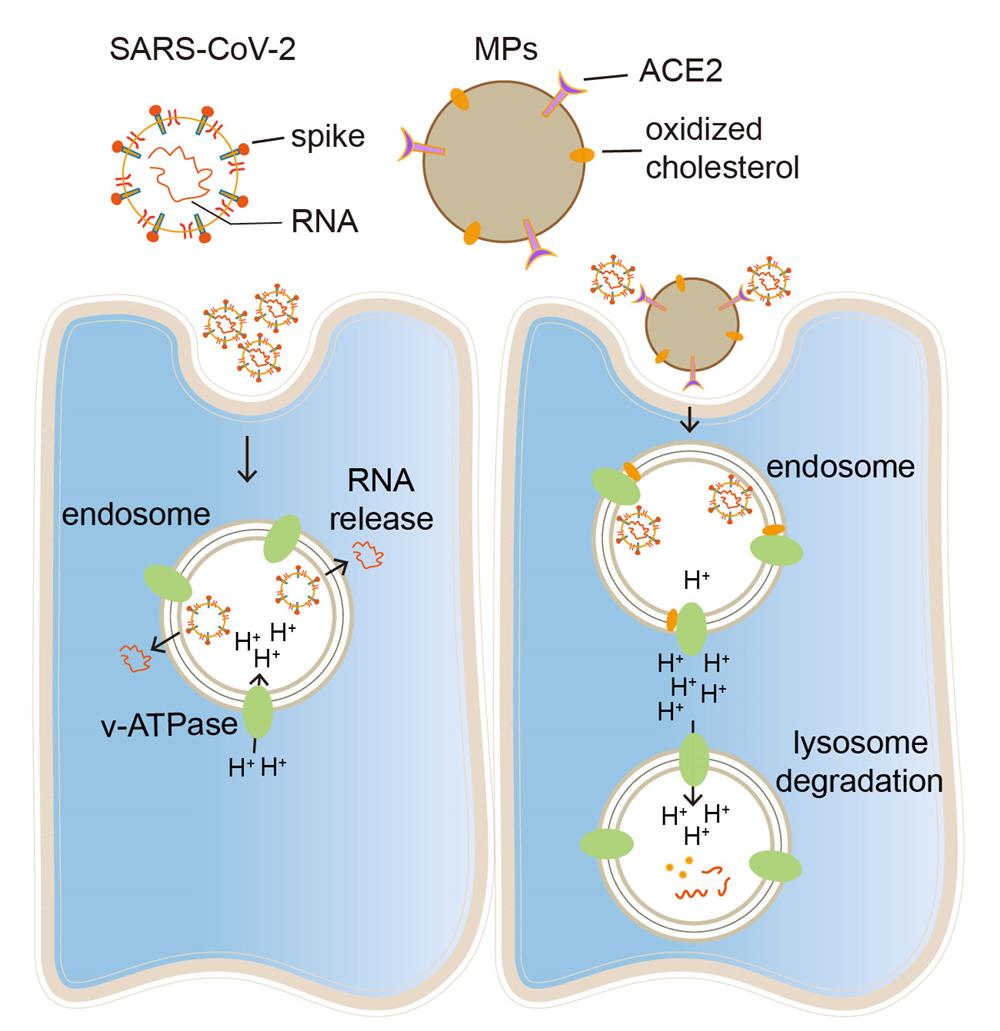
Anatomically, the respiratory trachea branches off into two bronchi, and the bronchi are further divided into bronchioles and respiratory bronchioles, which end in alveoli. The bronchi and bronchioles are mainly composed of ciliated epithelial cells and mucin-producing goblet cells, with the cilia sweeping off particle-trapping mucus. Thus, a virus invading the bronchial or bronchiolar epithelium readily causes cough with sputum. The clinical symptom of dry cough in SARS-CoV-2-infected patients suggests that this virus mainly invades the alveoli rather than the bronchioles or bronchi. The alveolus is a tiny, thin-walled, capillary-rich sac structure where alveolar macrophages reside to clear billions of inhaled particles, allergens, and microbes daily. Inhaled Delta variant of SARS-CoV-2 gain access to and become trapped in the lining fluid of the alveoli, which hinders the infection of underlying alveolar epithelial cells but allows alveolar macrophages (AMs) to phagocytose the virus into the endosomes. While endosomal enzyme cathepsin L (CTSL) catalyzes viral spike protein for the release of viral RNA into the cytosol and further viral replication, this process relies on an acidic endosomal pH by inducing substrate protonation. Unlike M1-polarized AMs, M2 AMs, the common form in the alveoli, have a more basic endosomal pH, thus limiting SARS-CoV-2 spread. However, Delta mutations with T478K, P681R and L452R facilitate spike protein auto-protonation by providing amino groups. In this study, we demonstrated that CTSL still can effectively catalyzes Delta spike protein rather than other variants (D614G, Beta or Gamma) in M2 AMs, leading to rapid synthesis of Delta progenies, which are released and cause ultrafast spread.
Cellular microparticles are vesicular plasma membrane fragments with a diameter of 0.1~1 μm that are shed by cells in response to various physiological and artificial stimuli. We found that ACE2-overexpressing A549 cell-derived microparticles (AO-MPs) are a potential therapeutic agent against SARS-CoV-2 infection. Intranasally administered AO-MPs dexterously navigate the anatomical and biological features of the lungs to enter the alveoli and are taken up by alveolar macrophages (AMs). Then, AO-MPs increase the endosomal pH but decrease the lysosomal pH in AMs, thus escorting bound SARS-CoV-2 from phago-endosomes to lysosomes for degradation. This pH regulation is attributable to oxidized cholesterol, which is enriched in AO-MPs and translocated to endosomal membranes, thus interfering with proton pumps and impairing endosomal acidification. In addition to promoting viral degradation, AO-MPs also inhibit the proinflammatory phenotype of AMs, leading to increased treatment efficacy in a SARS-CoV-2-infected mouse model without side effects. These findings highlight the potential use of AO-MPs to treat SARS-CoV-2-infected patients and showcase the feasibility of MP therapies for combatting emerging respiratory viruses in the future.
This research works can be mainly completed by the team of Professor Huang Bo from the Institute of Basic Medicine of the Chinese Academy of Medical Sciences and the team of Professor Qin Chuan from the Institute of Medical Laboratory Animals of the Chinese Academy of Medical Sciences, meanwhile the project is funded by the Medical and Health Technology Innovation Project of the Chinese Academy of Medical Sciences. This project was funded by the Medical and Health Technology Innovation Project of the Chinese Academy of Medical Sciences.
Links:
https://www.nature.com/articles/s41392-021-00845-4
https://www.nature.com/articles/s41423-021-00813-6
The SARS-COV-2 has caused a large number of infections since it was discovered at the end of 2019. As the increase in the number of infections and the continuation of the epidemic, SARS-CoV-2 continues to evolve and mutate, resulting in numerous virus variants, some of which have significantly improved transmission capacity and pathogenicity. At the meantime, there are also immune escape phenomena which were found in some strains, then these mutant strains have aroused the attention of public health departments and the public. Presently, the SARS-CoV-2 Delta variant (B.1.617.2) has significant replication and dissemination capabilities, and it is dominant in the world. It is very harmful to humans, but how the Delta variants with T478K, P681R and L452R mutations achieve their ultra-fast transmission is still unknown.
Anatomically, the respiratory trachea branches off into two bronchi, and the bronchi are further divided into bronchioles and respiratory bronchioles, which end in alveoli. The bronchi and bronchioles are mainly composed of ciliated epithelial cells and mucin-producing goblet cells, with the cilia sweeping off particle-trapping mucus. Thus, a virus invading the bronchial or bronchiolar epithelium readily causes cough with sputum. The clinical symptom of dry cough in SARS-CoV-2-infected patients suggests that this virus mainly invades the alveoli rather than the bronchioles or bronchi. The alveolus is a tiny, thin-walled, capillary-rich sac structure where alveolar macrophages reside to clear billions of inhaled particles, allergens, and microbes daily. Inhaled Delta variant of SARS-CoV-2 gain access to and become trapped in the lining fluid of the alveoli, which hinders the infection of underlying alveolar epithelial cells but allows alveolar macrophages (AMs) to phagocytose the virus into the endosomes. While endosomal enzyme cathepsin L (CTSL) catalyzes viral spike protein for the release of viral RNA into the cytosol and further viral replication, this process relies on an acidic endosomal pH by inducing substrate protonation. Unlike M1-polarized AMs, M2 AMs, the common form in the alveoli, have a more basic endosomal pH, thus limiting SARS-CoV-2 spread. However, Delta mutations with T478K, P681R and L452R facilitate spike protein auto-protonation by providing amino groups. In this study, we demonstrated that CTSL still can effectively catalyzes Delta spike protein rather than other variants (D614G, Beta or Gamma) in M2 AMs, leading to rapid synthesis of Delta progenies, which are released and cause ultrafast spread.
Cellular microparticles are vesicular plasma membrane fragments with a diameter of 0.1~1 μm that are shed by cells in response to various physiological and artificial stimuli. We found that ACE2-overexpressing A549 cell-derived microparticles (AO-MPs) are a potential therapeutic agent against SARS-CoV-2 infection. Intranasally administered AO-MPs dexterously navigate the anatomical and biological features of the lungs to enter the alveoli and are taken up by alveolar macrophages (AMs). Then, AO-MPs increase the endosomal pH but decrease the lysosomal pH in AMs, thus escorting bound SARS-CoV-2 from phago-endosomes to lysosomes for degradation. This pH regulation is attributable to oxidized cholesterol, which is enriched in AO-MPs and translocated to endosomal membranes, thus interfering with proton pumps and impairing endosomal acidification. In addition to promoting viral degradation, AO-MPs also inhibit the proinflammatory phenotype of AMs, leading to increased treatment efficacy in a SARS-CoV-2-infected mouse model without side effects. These findings highlight the potential use of AO-MPs to treat SARS-CoV-2-infected patients and showcase the feasibility of MP therapies for combatting emerging respiratory viruses in the future.
This research works can be mainly completed by the team of Professor Huang Bo from the Institute of Basic Medicine of the Chinese Academy of Medical Sciences and the team of Professor Qin Chuan from the Institute of Medical Laboratory Animals of the Chinese Academy of Medical Sciences, meanwhile the project is funded by the Medical and Health Technology Innovation Project of the Chinese Academy of Medical Sciences. This project was funded by the Medical and Health Technology Innovation Project of the Chinese Academy of Medical Sciences.


Links:
https://www.nature.com/articles/s41392-021-00845-4
https://www.nature.com/articles/s41423-021-00813-6


