Memory T cells also have ammonia detoxification: another key to solve the mystery of memory
Source:Ke Tang
2022-12-29
The metabolism of sugar, lipid and protein is the basis of all life activities, but at the same time, it inevitably produces substances that endanger life. Carbohydrate and lipid metabolism, which providing energy for life, meanwhile forms toxic free radicals. Nevertheless, the catabolism of proteins releases the chronic cytotoxic molecule ammonia (NH3). The cells of various tissues and organs have a powerful antioxidant (free radical scavenging) function, which has been carried out extensive research, but the study of ammonia clearance is very limited except for the liver cells convert NH3 to urea for detoxification. On December 6, 2022, the journal "Nature Immunology", published the latest research paper "Ammonia detoxification promotes CD8+ T cell memory development by urea and citrulline cycles" by Prof. Huang Bo from Institute of Basic Medicine Chinese Academy of Medical Sciences / Huazhong University of Science and Technology. This study reveals that urea cycle metabolism plays an important role in maintaining the development and long-term survival of memory T cells, and illustrates the basic immunological issue of T cell memory formation from a completely new metabolic pathway.

Memory T (Tm) cells are typical long-lived cells, which can exist in the body for several months to few years or much for entire life. The formation and maintenance of memory T cells are the essential of vaccine protection, tumor immunotherapy and antiviral infection in the body. But how to dissect the root cause of the long lifespan of memory T cells is still a considerable problem for immunologists. Bo Huang group has a long-term commitment to reveal how the memory T cells to maintain long life and the key metabolic characteristics in it. In 2018, the group found memory T cells long-term survival which is dependent on the unique gluconeogenesis-glycogen metabolism metabolic pattern. Tm can sustain high levels of reduced glutathione and timely remove intracellular reactive oxygen species (ROS), through high expression of gluconeogenesis pathway key rate-limiting enzyme phosphoenolpyruvate carboxyl kinase PCK1 promoting the synthesis of glycogen, meanwhile pentose phosphate pathway producing prototype NADPH. Thus, Tm could maintain the long-term survival ability. It is well known that Tm is derived from effector T cells. So why does the expression of PCK1 differ between Tm and Teff? With the deeply research, the research group found that the ketone body metabolism existed in the memory T cells. Unlike conventional energy supply of ketone body, β-hydroxybutyric acid produced by Tm cells promotes the high expression of PCK1, which engages T cells memory maintenance and long-term survival, related studies were published on journal "nature cell biology" in 2018 and 2020 (Nat Cell Biol.2018; 20:21-27; Nat Cell Biol. 2020; 22:18-25).
It is well known that cells need the energy molecule ATP to maintain life activities. Glucose and fatty acid oxidation are the major source of ATP generation. In addition, amino acids can also be oxidized to provide or regulate energy generation after deamination. However, ROS and ammonia (NH3, as byproducts during cellular ATP production) are also inevitably formed, meanwhile the substances are cytotoxic, weakening cell life span. Thus, long-lived cells must utilize efficient mechanisms to clear ROS and ammonia for prolong survival.
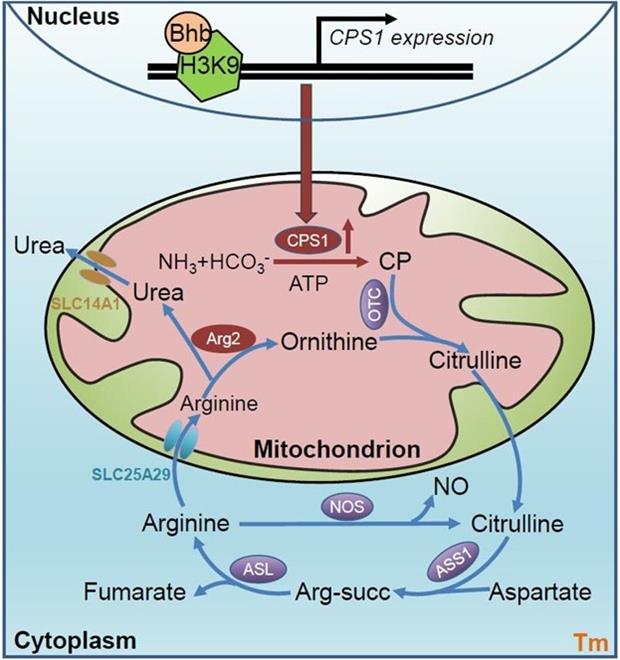
In the previous study, the research team has revealed how memory T cells clear ROS, but whether CD8+ Tm cells can remove toxic ammonia by itself metabolism remains a mystery. Deamination of amino acids is the major source of intracellular ammonia and the reaction process has two main steps: the first step is the generation of glutamate or glutamine mediated by transamination, and the second step is the deamination of glutamate or glutamine. It is widely believed that ammonia is processed in the liver from the urea cycle, where hepatocyte absorbs ammonia from the Peripheral circulation and use carbamoyl phosphate synthetase 1 (CPS1) to catalyze ammonia and bicarbonate (HCO3-) and form carbamoyl phosphate (CP) in mitochondria. What's more, CP reacts with ornithine to generate citrulline by ornithine carbamoyl phosphotransferase (OTC). After citrulline enters the cytosol, arginine succinate synthase 1 (ASS1) combines to produce arginine, then arginine lyase (ASL) metabolizes it to arginine and fumaria acid, furthermore arginase 1 (ARG1) hydrolyzes arginine to ornithine and urea, at last, ornithine enters the mitochondria to complete a cycle. The traditional views are that the urea cycle only exists in the liver. However, we determined for the first time that urea cycle existed in memory T cells and played an important role, by establishing an in vivo adoptive model of antigen-specific memory T cells, with isotope tracer technology and ultra-high resolution liquid chromatography-mass spectrometry analysis. Furthermore, we verified that the urea cycle was necessary for memory maintenance of Tm cells through a series of animal models and in vitro mechanism analysis. In the following study, the authors further found it different that the traditional urea cycle generates urea through arginase 1, but CD8+ Tm cells use arginase 2, localized in mitochondria, to catalyze producing urea from arginine. And further studied that arginine and urea shuttle back and forth in mitochondria through two solute protein transporters, SLC25A29 and SLC14A1. Mechanistic studies also found that CD8+ Tm cells used the citrulline cycle to catabolize ammonia besides the urea cycle, which worked together with the urea cycle. In addition, the authors verified that the gene expression of urea cycle key enzyme Cps1 are essential for ammonia handling and memory maintenance in Tm cells using multiple animal models, and further found that β-hydroxybutyrylation in the Cps1 transcription promoter region is the key to induce Cps1 expression by epigenetic modification. Eventually, Tm cells with high Cps1 expression also show a more efficient anti-tumor role in vivo tumor treatment model, which gives a new metabolic regulation idea for T cell adoptive transfer immunotherapy, and exhibits clear clinical transformation significance. The discoveries of this study will enable us to clarify the long-term survival mechanism of Tm cells from a new metabolic perspective, and will offer a new idea and transformation method of T cells-based tumor therapy.
This work was supported by the National Natural Science Foundation of China (81788101, 82071864, 82150103) and the Medical and Health Science and Technology Innovation Project of the Chinese Academy of Medical Sciences (2021-I2M-1-021). Associate Professor Ke Tang from the department of Biochemistry and Molecular Biology of the School of Basic Medicine, Huazhong University of science & technology is the first author, Associate Professor Huafeng Zhang from the department of pathology and Dr. Jinghui Deng are the Co-first authors of this paper. Professor Bo Huang is the corresponding author of this paper. Related information (DOI): 10.1038/s41590-022-01365-1

Memory T (Tm) cells are typical long-lived cells, which can exist in the body for several months to few years or much for entire life. The formation and maintenance of memory T cells are the essential of vaccine protection, tumor immunotherapy and antiviral infection in the body. But how to dissect the root cause of the long lifespan of memory T cells is still a considerable problem for immunologists. Bo Huang group has a long-term commitment to reveal how the memory T cells to maintain long life and the key metabolic characteristics in it. In 2018, the group found memory T cells long-term survival which is dependent on the unique gluconeogenesis-glycogen metabolism metabolic pattern. Tm can sustain high levels of reduced glutathione and timely remove intracellular reactive oxygen species (ROS), through high expression of gluconeogenesis pathway key rate-limiting enzyme phosphoenolpyruvate carboxyl kinase PCK1 promoting the synthesis of glycogen, meanwhile pentose phosphate pathway producing prototype NADPH. Thus, Tm could maintain the long-term survival ability. It is well known that Tm is derived from effector T cells. So why does the expression of PCK1 differ between Tm and Teff? With the deeply research, the research group found that the ketone body metabolism existed in the memory T cells. Unlike conventional energy supply of ketone body, β-hydroxybutyric acid produced by Tm cells promotes the high expression of PCK1, which engages T cells memory maintenance and long-term survival, related studies were published on journal "nature cell biology" in 2018 and 2020 (Nat Cell Biol.2018; 20:21-27; Nat Cell Biol. 2020; 22:18-25).
It is well known that cells need the energy molecule ATP to maintain life activities. Glucose and fatty acid oxidation are the major source of ATP generation. In addition, amino acids can also be oxidized to provide or regulate energy generation after deamination. However, ROS and ammonia (NH3, as byproducts during cellular ATP production) are also inevitably formed, meanwhile the substances are cytotoxic, weakening cell life span. Thus, long-lived cells must utilize efficient mechanisms to clear ROS and ammonia for prolong survival.
In the previous study, the research team has revealed how memory T cells clear ROS, but whether CD8+ Tm cells can remove toxic ammonia by itself metabolism remains a mystery. Deamination of amino acids is the major source of intracellular ammonia and the reaction process has two main steps: the first step is the generation of glutamate or glutamine mediated by transamination, and the second step is the deamination of glutamate or glutamine. It is widely believed that ammonia is processed in the liver from the urea cycle, where hepatocyte absorbs ammonia from the Peripheral circulation and use carbamoyl phosphate synthetase 1 (CPS1) to catalyze ammonia and bicarbonate (HCO3-) and form carbamoyl phosphate (CP) in mitochondria. What's more, CP reacts with ornithine to generate citrulline by ornithine carbamoyl phosphotransferase (OTC). After citrulline enters the cytosol, arginine succinate synthase 1 (ASS1) combines to produce arginine, then arginine lyase (ASL) metabolizes it to arginine and fumaria acid, furthermore arginase 1 (ARG1) hydrolyzes arginine to ornithine and urea, at last, ornithine enters the mitochondria to complete a cycle. The traditional views are that the urea cycle only exists in the liver. However, we determined for the first time that urea cycle existed in memory T cells and played an important role, by establishing an in vivo adoptive model of antigen-specific memory T cells, with isotope tracer technology and ultra-high resolution liquid chromatography-mass spectrometry analysis. Furthermore, we verified that the urea cycle was necessary for memory maintenance of Tm cells through a series of animal models and in vitro mechanism analysis. In the following study, the authors further found it different that the traditional urea cycle generates urea through arginase 1, but CD8+ Tm cells use arginase 2, localized in mitochondria, to catalyze producing urea from arginine. And further studied that arginine and urea shuttle back and forth in mitochondria through two solute protein transporters, SLC25A29 and SLC14A1. Mechanistic studies also found that CD8+ Tm cells used the citrulline cycle to catabolize ammonia besides the urea cycle, which worked together with the urea cycle. In addition, the authors verified that the gene expression of urea cycle key enzyme Cps1 are essential for ammonia handling and memory maintenance in Tm cells using multiple animal models, and further found that β-hydroxybutyrylation in the Cps1 transcription promoter region is the key to induce Cps1 expression by epigenetic modification. Eventually, Tm cells with high Cps1 expression also show a more efficient anti-tumor role in vivo tumor treatment model, which gives a new metabolic regulation idea for T cell adoptive transfer immunotherapy, and exhibits clear clinical transformation significance. The discoveries of this study will enable us to clarify the long-term survival mechanism of Tm cells from a new metabolic perspective, and will offer a new idea and transformation method of T cells-based tumor therapy.
This work was supported by the National Natural Science Foundation of China (81788101, 82071864, 82150103) and the Medical and Health Science and Technology Innovation Project of the Chinese Academy of Medical Sciences (2021-I2M-1-021). Associate Professor Ke Tang from the department of Biochemistry and Molecular Biology of the School of Basic Medicine, Huazhong University of science & technology is the first author, Associate Professor Huafeng Zhang from the department of pathology and Dr. Jinghui Deng are the Co-first authors of this paper. Professor Bo Huang is the corresponding author of this paper. Related information (DOI): 10.1038/s41590-022-01365-1



