Zhu Ping's team revealed a new mechanism of HLA-restricted autoimmune response induced by modified neoantigens in a published article in Science
Source:Zhu Ping
2023-04-07
Ankylosing spondylitis (AS) is a disease in which neoantigens in the human body disrupt immune tolerance, trigger antigen-specific immune responses, and cause multi-organ damage. Post-translational modifications (PTMs) are biological phenomena that can change the structure and function of proteins, or induce the production of neoantigens and cause autoimmune responses. The molecular mechanism of AS neoantigen formation and autoimmune response is still unknown, and it is urgent to develop a systematic method to analyze the protein modification profile of patients with AS and identify PTM related to autoreactive neoantigen production, and further deepen the understanding of the pathogenesis of autoimmune diseases such as AS.
On March 17, 2023, Zhu Ping's team from the Air Force Military Medical University published a long research paper entitled "Cysteine carboxyethylation generates neoantigens to induce HLA-restricted autoimmunity" in Science. This study has deciphered a new molecular mechanism of AS autoimmune disease.
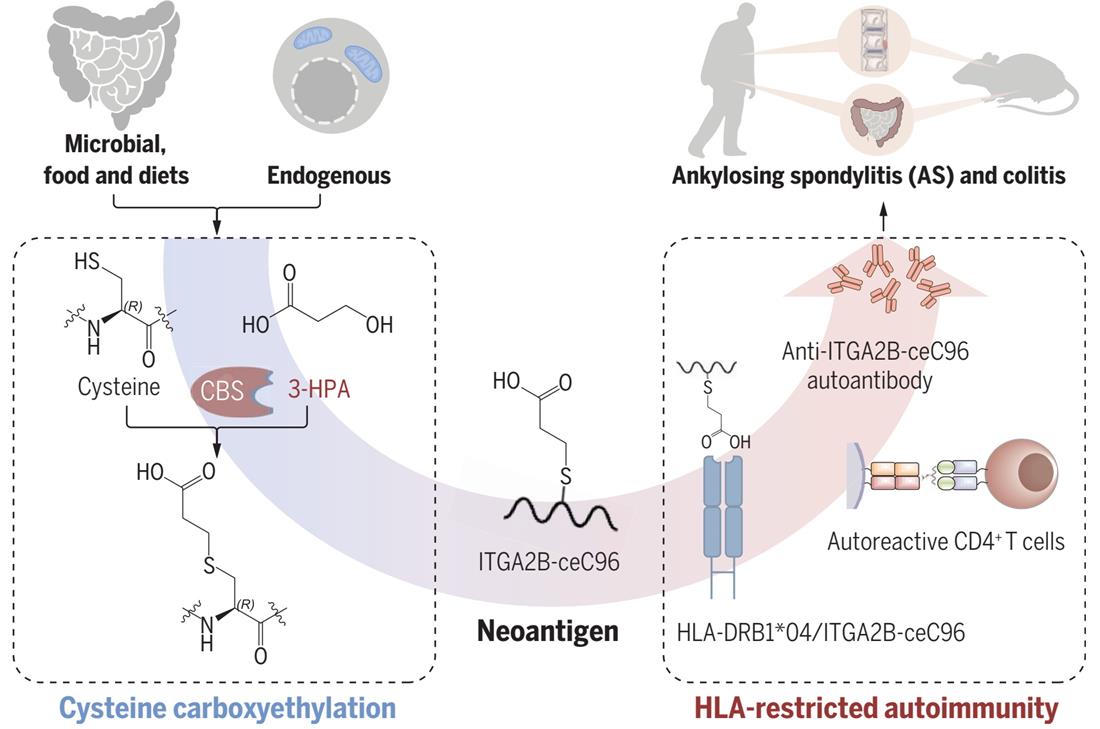
AS is an autoimmune disease and the onset of patients is significantly correlated with human leukocyte antigen (HLA) typing, such as HLA-B27, HLA-DRB1, etc.. However, the source of pathogenic autoantigens, the presentation of antigenic peptides, and the activation and response of antigen-specific T/B lymphocytes are unknown. To explore whether post-translational modifications are an important source of neoantigens in AS, the researchers developed a systematic open-search method to identify amino acid residues and derivatives in proteins. These amino acid residues, unlike translation products of genome-coded sequences, are potential post-translational modifications of proteins. First, possible amino acid derivatives in the proteome of AS patients were identified by an open search method, and a large number of non-coding amino acids (ncAAs) were analyzed, and the modification groups were expressed by deltamass or mass shifts. Among them, an amino acid derivative with a mass shift of 72.021 Da is associated with AS. Further analysis showed that the 72.021 Da mass shift in AS patients was highly enriched at the cysteine 96 of Integrin alpha 2B (ITGA2B). Verified by mass spectrometry and anti-PTM antibodies, the mass shift of 72.021Da was cysteine carboxyethylation. In vitro and in vivo experiments have shown that the metabolic substrate modified by cysteine carboxyethylation is 3-HPA, and the reaction can be catalyzed by cystathionine β synthase (CBS). 3-HPA is a metabolite released by gut microorganisms.
The researchers verified the AS disease-related potential modification spectra, screened for possible AS-associated PTM autoantigens (modified neoantigens), and studied the mechanism of neoantigens mediate pathogenesis in AS. The authors found that cysteine carboxyethylation modification induces lysosomal pathway degradation of ITGA2B molecules, inducing major histocompatibility complex (MHC) II-dependent CD4+ T cell responses. Fluorescence polarization and ELISA experiments showed that cysteine carboxyethylated modified peptide (ITGA2B-ceC96) could be presented to the cell membrane surface as a neoantigen by the MHC II complex HLA-DRA*01/HLA-DRB1*04. Patients with the HLA-DRB1*04 genotype produce neoantigen-specific autoantibodies and T cell responses that correlate with 3-HPA levels. 3-HPA administration combined with ITGA2B-ceC96 antigenic peptide immune HLA-DR4 transgenic mice could be induced to reproduce AS characteristic inflammatory bowel disease and spinal lesions.
Cysteine carboxyethylation modification is a novel protein modification form caused by the metabolite 3-HPA in vivo, and the carboxyethylated modified ITGA2B can induce AS to produce modified neoantigens and undergo specific autoimmune responses. This study reveals for the first time the pathogenic mechanism and new theory of "environment-metabolite-PTM-neoantigen" of autoimmune disease, which lays a foundation for the study of the etiology and pathology of metabolite modification to form neoantigens and induce autoimmune diseases, and is of great significance for the establishment of specific screening and targeted treatment strategies for immune diseases such as AS.
Science published a perspective of this study in the same issue by Laura Santambrogio, an immunologist at Weill Cornell Medical College in the United Kingdom, "Autoimmunity to the modified self", specifically noting that this study revealed that protein post-translational modifications can break tolerance to their own proteome, that is, PTMs pose a danger for the development of autoimmune diseases because they change the protein “self” sequence.
Professor Zhu Ping of Department of Clinical Immunology, Xijing Hospital, and National Translational Science, Center for Molecular Medicine, Fourth Military Medical University, is the corresponding author of the paper. Professor Yang Jing-Hua of Zhengzhou University and Professor Chen Liang of Shanghai University are the corresponding authors. Dr. Zhai Yue from Department of Clinical Immunology, Xijing Hospital, and Department of Cell Biology of National Translational Science Center for Molecular Medicine, Fourth Military Medical University is the first author of the paper.
This research was supported by the National Natural Science Foundation of China, the National Key Basic Research and Development Plan of the Ministry of Science and Technology (973 Program), the Young Elite Scientists Sponsorship Program by CAST, and the Tai-Shan Scholarship of Shandong Province.
Links: https://www.science.org/doi/10.1126/science.abg2482
On March 17, 2023, Zhu Ping's team from the Air Force Military Medical University published a long research paper entitled "Cysteine carboxyethylation generates neoantigens to induce HLA-restricted autoimmunity" in Science. This study has deciphered a new molecular mechanism of AS autoimmune disease.

AS is an autoimmune disease and the onset of patients is significantly correlated with human leukocyte antigen (HLA) typing, such as HLA-B27, HLA-DRB1, etc.. However, the source of pathogenic autoantigens, the presentation of antigenic peptides, and the activation and response of antigen-specific T/B lymphocytes are unknown. To explore whether post-translational modifications are an important source of neoantigens in AS, the researchers developed a systematic open-search method to identify amino acid residues and derivatives in proteins. These amino acid residues, unlike translation products of genome-coded sequences, are potential post-translational modifications of proteins. First, possible amino acid derivatives in the proteome of AS patients were identified by an open search method, and a large number of non-coding amino acids (ncAAs) were analyzed, and the modification groups were expressed by deltamass or mass shifts. Among them, an amino acid derivative with a mass shift of 72.021 Da is associated with AS. Further analysis showed that the 72.021 Da mass shift in AS patients was highly enriched at the cysteine 96 of Integrin alpha 2B (ITGA2B). Verified by mass spectrometry and anti-PTM antibodies, the mass shift of 72.021Da was cysteine carboxyethylation. In vitro and in vivo experiments have shown that the metabolic substrate modified by cysteine carboxyethylation is 3-HPA, and the reaction can be catalyzed by cystathionine β synthase (CBS). 3-HPA is a metabolite released by gut microorganisms.
The researchers verified the AS disease-related potential modification spectra, screened for possible AS-associated PTM autoantigens (modified neoantigens), and studied the mechanism of neoantigens mediate pathogenesis in AS. The authors found that cysteine carboxyethylation modification induces lysosomal pathway degradation of ITGA2B molecules, inducing major histocompatibility complex (MHC) II-dependent CD4+ T cell responses. Fluorescence polarization and ELISA experiments showed that cysteine carboxyethylated modified peptide (ITGA2B-ceC96) could be presented to the cell membrane surface as a neoantigen by the MHC II complex HLA-DRA*01/HLA-DRB1*04. Patients with the HLA-DRB1*04 genotype produce neoantigen-specific autoantibodies and T cell responses that correlate with 3-HPA levels. 3-HPA administration combined with ITGA2B-ceC96 antigenic peptide immune HLA-DR4 transgenic mice could be induced to reproduce AS characteristic inflammatory bowel disease and spinal lesions.
Cysteine carboxyethylation modification is a novel protein modification form caused by the metabolite 3-HPA in vivo, and the carboxyethylated modified ITGA2B can induce AS to produce modified neoantigens and undergo specific autoimmune responses. This study reveals for the first time the pathogenic mechanism and new theory of "environment-metabolite-PTM-neoantigen" of autoimmune disease, which lays a foundation for the study of the etiology and pathology of metabolite modification to form neoantigens and induce autoimmune diseases, and is of great significance for the establishment of specific screening and targeted treatment strategies for immune diseases such as AS.

Science published a perspective of this study in the same issue by Laura Santambrogio, an immunologist at Weill Cornell Medical College in the United Kingdom, "Autoimmunity to the modified self", specifically noting that this study revealed that protein post-translational modifications can break tolerance to their own proteome, that is, PTMs pose a danger for the development of autoimmune diseases because they change the protein “self” sequence.

Professor Zhu Ping of Department of Clinical Immunology, Xijing Hospital, and National Translational Science, Center for Molecular Medicine, Fourth Military Medical University, is the corresponding author of the paper. Professor Yang Jing-Hua of Zhengzhou University and Professor Chen Liang of Shanghai University are the corresponding authors. Dr. Zhai Yue from Department of Clinical Immunology, Xijing Hospital, and Department of Cell Biology of National Translational Science Center for Molecular Medicine, Fourth Military Medical University is the first author of the paper.
This research was supported by the National Natural Science Foundation of China, the National Key Basic Research and Development Plan of the Ministry of Science and Technology (973 Program), the Young Elite Scientists Sponsorship Program by CAST, and the Tai-Shan Scholarship of Shandong Province.
Links: https://www.science.org/doi/10.1126/science.abg2482


