Prof. Jiahuai Han's group reports ribosome-rescuer PELO catalyzes the oligomeric assembly and activation of NLR family proteins
Source:Zhang-Hua Yang
2023-06-06
NOD-like receptor (NLR) family members are evolution-derived intracellular pattern recognition receptors (PRRs) for various pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). It is currently believed that after NLRs sense and recognize corresponding pattern molecules, they undergo self-oligomeric assembly to form large signaling complexes such as inflammasome (e.g., NLRP3, NLRC4) and nodosome (e.g., NOD2). These multiple-protein complexes activate various downstream signaling pathways including the NF-κB pathway, MAPK pathway, and pyroptotic cell death, mediating a series of immune-inflammatory cascades. This is one of the most fundamental innate immune defense responses and plays a crucial role in the clearance of pathogenic infections and endogenous danger signals. In the human body, abnormal activation of NLR caused by gene mutations has been found to be involved in the pathological processes of various inflammatory diseases such as sepsis and inflammatory bowel disease.
Structurally, NLR family proteins all contain a nucleotide-binding oligomerization domain (NOD or NACHT), which is crucial for the activation of NLRs. Numerous studies have shown that self-oligomerization mediated by the NACHT domain is a common feature and the primary mode of activation for NLR proteins. However, the mechanism of oligomeric assembly and activation of NLR family proteins is currently not well understood.
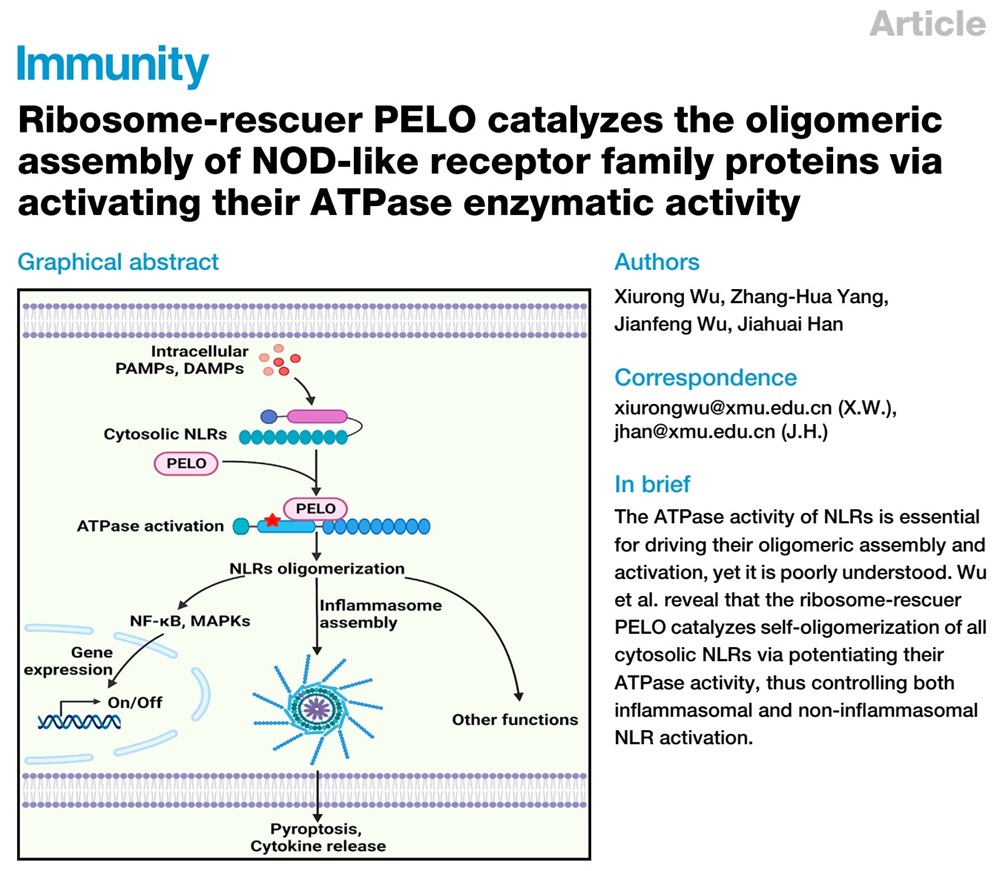
On May 9, 2023, a research article titled "Ribosome-rescuer PELO catalyzes the oligomeric assembly of NOD-like receptor family proteins via activating their ATPase enzymatic activity" was published in Immunity by the team led by Prof. Jiahuai Han from the State Key Laboratory of Cellular Stress Biology, School of Life Sciences/and School of Medicine at Xiamen University. The study revealed the critical role of ribosome quality control factor PELO in efficiently activating the ATPase activity of NLR proteins, thereby controlling their oligomeric assembly and activation, and participating in the regulation of various immune and inflammatory responses mediated by the NLR family proteins.
PELO is an evolutionarily conserved protein that is involved in ribosome-associated quality control (RQC) and mediates the disassembly and recycling of stalled ribosomes through ribosome rescue. PELO has been reported to play important roles in various physiological processes such as embryonic development, spermatogenesis, epidermal homeostasis, and neurodevelopment. However, its role in cellular stress responses, including immune responses, has remained unknown. The team led by Jiahuai Han previously identified PELO through forward genetic screening in Drosophila and found its involvement in antiviral innate immunity. Subsequent studies have shown that homologs of PELO in plants are also associated with antiviral immune responses, indicating the highly conserved nature of PELO's involvement in immune responses.
In this study, the researchers unexpectedly found PELO is an interacting protein of NLRP3 inflammasome by quantitative mass spectrum analysis. The researcher confirmed the direct interaction between PELO and NLRP3 and found that this interaction is mediated by the NACHT and LRR domains of NLRP3. As the NACHT and LRR domains are common domains among NLR family proteins, the researchers further analyzed and discovered that PELO can specifically interact with all cytoplasmic NLR family proteins. The researchers validated the involvement of PELO in the regulation of NLR family protein-mediated innate immune responses, such as the activation of NF-κB and MAPK signaling pathways mediated by NOD2 and the activation of inflammasomes mediated by NLRP3, NLRC4, and NLRR6, in various cell models and mouse models. Further cellular experiments revealed that PELO controls the oligomeric assembly of NLR proteins by directly binding to NLR proteins. NLR proteins belong to the STAND (signal transduction ATPases with numerous domains) ATPase family, and their NACHT domains contain conserved ATP binding and hydrolysis domains, which are crucial for the assembly and activation of NLR proteins. Through in vitro ATPase activity assays, the researchers found that PELO efficiently activates the ATPase activity of all the cytosolic NLR family proteins. Furthermore, the researchers established an in vitro assembly system for NLRC4 inflammasome and confirmed that PELO controls the oligomeric assembly and activation of NLRC4 by directly activating its ATPase activity.
In summary, this study reveals the novel immunological function of PELO and identifies it as a common interacting factor for all cytoplasmic NLR proteins. PELO efficiently activates the ATPase activity of NLR proteins to control their oligomeric assembly and activation, and this mechanism should propose a new paradigm for the activation of NLR family proteins.
The first authors of the article are Dr. Xiurong Wu from the School of Life Sciences at Xiamen University and Dr. Zhang-Hua Yang from the School of Medicine at Xiamen University. Prof. Jiahuai Han and Dr. Xiurong Wu are the corresponding authors of the article.
Original article link: https://doi.org/10.1016/j.immuni.2023.02.014
Structurally, NLR family proteins all contain a nucleotide-binding oligomerization domain (NOD or NACHT), which is crucial for the activation of NLRs. Numerous studies have shown that self-oligomerization mediated by the NACHT domain is a common feature and the primary mode of activation for NLR proteins. However, the mechanism of oligomeric assembly and activation of NLR family proteins is currently not well understood.

On May 9, 2023, a research article titled "Ribosome-rescuer PELO catalyzes the oligomeric assembly of NOD-like receptor family proteins via activating their ATPase enzymatic activity" was published in Immunity by the team led by Prof. Jiahuai Han from the State Key Laboratory of Cellular Stress Biology, School of Life Sciences/and School of Medicine at Xiamen University. The study revealed the critical role of ribosome quality control factor PELO in efficiently activating the ATPase activity of NLR proteins, thereby controlling their oligomeric assembly and activation, and participating in the regulation of various immune and inflammatory responses mediated by the NLR family proteins.
PELO is an evolutionarily conserved protein that is involved in ribosome-associated quality control (RQC) and mediates the disassembly and recycling of stalled ribosomes through ribosome rescue. PELO has been reported to play important roles in various physiological processes such as embryonic development, spermatogenesis, epidermal homeostasis, and neurodevelopment. However, its role in cellular stress responses, including immune responses, has remained unknown. The team led by Jiahuai Han previously identified PELO through forward genetic screening in Drosophila and found its involvement in antiviral innate immunity. Subsequent studies have shown that homologs of PELO in plants are also associated with antiviral immune responses, indicating the highly conserved nature of PELO's involvement in immune responses.
In this study, the researchers unexpectedly found PELO is an interacting protein of NLRP3 inflammasome by quantitative mass spectrum analysis. The researcher confirmed the direct interaction between PELO and NLRP3 and found that this interaction is mediated by the NACHT and LRR domains of NLRP3. As the NACHT and LRR domains are common domains among NLR family proteins, the researchers further analyzed and discovered that PELO can specifically interact with all cytoplasmic NLR family proteins. The researchers validated the involvement of PELO in the regulation of NLR family protein-mediated innate immune responses, such as the activation of NF-κB and MAPK signaling pathways mediated by NOD2 and the activation of inflammasomes mediated by NLRP3, NLRC4, and NLRR6, in various cell models and mouse models. Further cellular experiments revealed that PELO controls the oligomeric assembly of NLR proteins by directly binding to NLR proteins. NLR proteins belong to the STAND (signal transduction ATPases with numerous domains) ATPase family, and their NACHT domains contain conserved ATP binding and hydrolysis domains, which are crucial for the assembly and activation of NLR proteins. Through in vitro ATPase activity assays, the researchers found that PELO efficiently activates the ATPase activity of all the cytosolic NLR family proteins. Furthermore, the researchers established an in vitro assembly system for NLRC4 inflammasome and confirmed that PELO controls the oligomeric assembly and activation of NLRC4 by directly activating its ATPase activity.
In summary, this study reveals the novel immunological function of PELO and identifies it as a common interacting factor for all cytoplasmic NLR proteins. PELO efficiently activates the ATPase activity of NLR proteins to control their oligomeric assembly and activation, and this mechanism should propose a new paradigm for the activation of NLR family proteins.
The first authors of the article are Dr. Xiurong Wu from the School of Life Sciences at Xiamen University and Dr. Zhang-Hua Yang from the School of Medicine at Xiamen University. Prof. Jiahuai Han and Dr. Xiurong Wu are the corresponding authors of the article.
Original article link: https://doi.org/10.1016/j.immuni.2023.02.014


