Prof. Shu Zhu’s group reports how intestinal epithelial cells recognize the food antigen and mediate the immune tolerance to food
Source:Shu Zhu
2023-07-27
A central question for immunology is: How does the host immune system discriminate the self and non-self molecules. The host has developed central tolerance or peripheral tolerance to prevent the immune system from attacking self-antigens or foreign harmless antigens from commensals and food, respectively. The loss of normal immune tolerance to food is most obviously associated with food allergies (e.g., peanut, egg, milk, etc.) and celiac diseases. It is unclear how protective immunity is induced against pathogens while still maintaining immune tolerance to food, and a deeper understanding of these processes should facilitate development of treatments against infection, inflammation, and allergy conditions in the intestine.
On June 15, 2023, Shu Zhu’s group of University of Science and Technology of China published a research paper entitled “Gasdermin D licenses MHCII induction to maintain food tolerance in the small intestine” in Cell. It was found that genetic knockout of the pyroptosis executioner protein Gasdermin D (GSDMD) in intestinal epithelial cells (IECs) disrupts immune tolerance to food in the small intestine, and IECs accumulate a less recognized 13kD N-terminal fragment of GSDMD that is cleaved by CASPASE (CASP)-3 and CASP-7 in response to dietary antigens. Unlike the 30kD GSDMD cleavage fragment that executes pyroptosis by translocating from the cytosol and rupturing cell membranes, this 13kD N-terminal GSDMD cleavage fragment translocates to the nucleus, where it induces the transcription of CIITA and MHCII molecules in IECs, which in turn induces the Type 1 regulatory T cells in upper small intestine to regulate food tolerance.
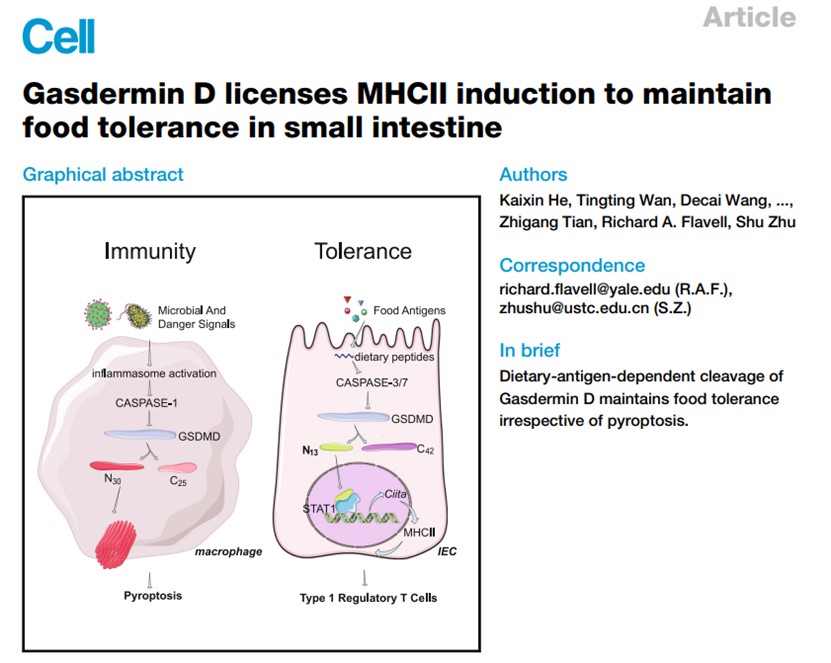
In this work, they first discovered a totally new function for Gasdermin D, the extremely well-studied pyroptosis executioner protein of innate immunity. Very briefly, our detection of a previously unreported 13kD N-terminal Gasdermin D cleavage fragment in intestinal epithelial cells motivated our study, which eventually led to our elucidation of the mechanism through which mammalian intestines simultaneously 1) allow immune tolerance that enables uptake of food and maintenance of commensal microbiota while 2) preserving immunity against pathogens.
Specifically, by using a variety of knockout, edited knock-in, conditional knockout cell-type-specific mouse lines, and cleavage resistant-mutant mice, they show that dietary antigens but not commensals or other immune perturbations induce previously undescribed 13/42 kD cleavage fragments of Gasdermin D in intestinal epithelial cells, and demonstrate that the 13 kD fragment is required for maintaining immune tolerance to food.
Biochemically, they found that this specific form of Gasdermin D cleavage is catalyzed by CASPASE-3/7, and they harnessed this discovery with extensive in vivo experiments using a caspase inhibitor to add further specificity to our understanding of how the 13 kD GSDMD cleavage fragment controls IEC functions and immune tolerance to food.
Excitingly, they demonstrate that the dietary-antigen-induced, nuclear-localized 13 kD GSDMD cleavage fragment induces multiple MHCII molecules, as well as the known master MHCII regulator CIITA in intestinal epithelial cells. And they also show that the 13 kD GSDMD cleavage fragment promotes the expansion of the CD4+IL-10+Foxp3- T regulatory cell (Tr1) population specifically in the upper small intestine, the region of the intestine where dietary antigens are prevalent.
Thus, when viewed alongside previous studies showing how danger signals and pathogens induce the cell-death-causing cleavage of Gasdermin D, the discovery of this new Gasdermin D mechanism suggests that the differential cleavage of GSDMD may serve as regulatory hub which controls innate immunity versus food tolerance in the small intestine. These insights concerning GSDMD's divergent roles in immunity-versus-tolerance deepen our understanding of innate immunity generally and may facilitate the development of new therapies to treat food allergies.
Dr. Zhu Shu of USTC, and Dr. Richard A. Flavell of Yale University, are co-corresponding authors of the paper. The work was funded by the National Natural Science Foundation of China, the National Key R&D Program of China, and the Chinese Academy of Sciences.
Article link: https://doi.org/10.1016/j.cell.2023.05.027
On June 15, 2023, Shu Zhu’s group of University of Science and Technology of China published a research paper entitled “Gasdermin D licenses MHCII induction to maintain food tolerance in the small intestine” in Cell. It was found that genetic knockout of the pyroptosis executioner protein Gasdermin D (GSDMD) in intestinal epithelial cells (IECs) disrupts immune tolerance to food in the small intestine, and IECs accumulate a less recognized 13kD N-terminal fragment of GSDMD that is cleaved by CASPASE (CASP)-3 and CASP-7 in response to dietary antigens. Unlike the 30kD GSDMD cleavage fragment that executes pyroptosis by translocating from the cytosol and rupturing cell membranes, this 13kD N-terminal GSDMD cleavage fragment translocates to the nucleus, where it induces the transcription of CIITA and MHCII molecules in IECs, which in turn induces the Type 1 regulatory T cells in upper small intestine to regulate food tolerance.

In this work, they first discovered a totally new function for Gasdermin D, the extremely well-studied pyroptosis executioner protein of innate immunity. Very briefly, our detection of a previously unreported 13kD N-terminal Gasdermin D cleavage fragment in intestinal epithelial cells motivated our study, which eventually led to our elucidation of the mechanism through which mammalian intestines simultaneously 1) allow immune tolerance that enables uptake of food and maintenance of commensal microbiota while 2) preserving immunity against pathogens.
Specifically, by using a variety of knockout, edited knock-in, conditional knockout cell-type-specific mouse lines, and cleavage resistant-mutant mice, they show that dietary antigens but not commensals or other immune perturbations induce previously undescribed 13/42 kD cleavage fragments of Gasdermin D in intestinal epithelial cells, and demonstrate that the 13 kD fragment is required for maintaining immune tolerance to food.
Biochemically, they found that this specific form of Gasdermin D cleavage is catalyzed by CASPASE-3/7, and they harnessed this discovery with extensive in vivo experiments using a caspase inhibitor to add further specificity to our understanding of how the 13 kD GSDMD cleavage fragment controls IEC functions and immune tolerance to food.
Excitingly, they demonstrate that the dietary-antigen-induced, nuclear-localized 13 kD GSDMD cleavage fragment induces multiple MHCII molecules, as well as the known master MHCII regulator CIITA in intestinal epithelial cells. And they also show that the 13 kD GSDMD cleavage fragment promotes the expansion of the CD4+IL-10+Foxp3- T regulatory cell (Tr1) population specifically in the upper small intestine, the region of the intestine where dietary antigens are prevalent.
Thus, when viewed alongside previous studies showing how danger signals and pathogens induce the cell-death-causing cleavage of Gasdermin D, the discovery of this new Gasdermin D mechanism suggests that the differential cleavage of GSDMD may serve as regulatory hub which controls innate immunity versus food tolerance in the small intestine. These insights concerning GSDMD's divergent roles in immunity-versus-tolerance deepen our understanding of innate immunity generally and may facilitate the development of new therapies to treat food allergies.
Dr. Zhu Shu of USTC, and Dr. Richard A. Flavell of Yale University, are co-corresponding authors of the paper. The work was funded by the National Natural Science Foundation of China, the National Key R&D Program of China, and the Chinese Academy of Sciences.
Article link: https://doi.org/10.1016/j.cell.2023.05.027


