Prof. Ji-hang Yuan’s group from Naval Medical University reveals a new mechanism of phagocytosis of tumor cells by Kupffer cells during tumor liver metastasis
Source:Ji-hang Yuan
2023-10-19
Adaptive immune checkpoint inhibitors (including CTLA-4, PD-L1, and PD-1 antibodies) have shown certain therapeutic effects in the treatment of some cancers, but the patient response rate is low. Innate immune cells provide nonspecific defense against tumor cells, especially for phagocytosis and clearance of tumor cells. The phagocytic effect of tumor cells is influenced by pro-phagocytosis ("eat me", such as Fc receptors) and anti-phagocytosis ("don't eat me", such as CD47-SIRP α, PD-1-PD-L1) signals. Various phagocytes and tumor cells use different signal combinations to regulate phagocytosis.
The liver is a highly vascularized target organ for metastasis. Various tumor cells, such as liver cancer, colorectal cancer, pancreatic cancer, melanoma, lung cancer, etc., are prone to liver metastasis. Kupffer cells, the hepatic resident macrophages, engulf these disseminated malignant cells and as such serve as the first line of defense. However, the specific "eat me" or "don't eat me" signals that mediate Kupffer cell recognition and phagocytosis of tumor cells are still unclear. Identifying the signals regulating phagocytosis in Kupffer cells can provide a theoretical basis for developing new strategies to enhance Kupffer phagocytosis and limit tumor liver metastasis.
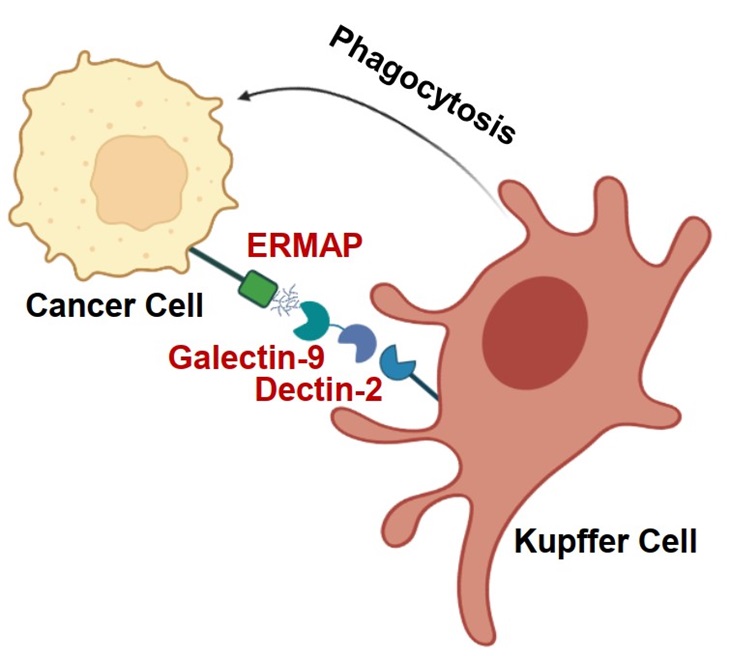
On October 9, 2023, Prof. Ji-hang Yuan’s group from Naval Medical University published a research paper online in Nature Immunology entitled "The ligation between ERMAP, galectin-9 and dectin-2 promotes Kupffer cell phagocytosis and anti-tumor immunity". This study found that the transmembrane protein ERMAP on tumor cells transmits the "eat me" signal to Kupffer cells through galectin-9/dectin-2, promotes Kupffer cells to engulf tumor cells, and inhibits tumor liver metastasis.
Yuan’s group conducted genome-wide CRISPR-cas9 knockout screening in an in vivo liver metastasis model, and unbiased identified genes regulating tumor liver metastasis. They found that gRNA targeting Ermap was enriched in liver metastatic tissues. Yuan’s group constructed tumor cell lines with ERMAP knocked out, knocked down, or overexpressed. In vivo liver metastasis experiments found that ERMAP inhibited tumor liver metastasis, but in vivo lung metastasis experiments found that ERMAP did not regulate tumor lung metastasis. In vitro functional experiments found that ERMAP did not regulate tumor cell proliferation, migration, and other behaviors, indicating that the specific role of ERMAP in liver metastasis was depends on the liver tissue microenvironment. Yuan’s group further found that ERMAP specifically bound to hepatic resident macrophage Kupffer cells and promoted Kupffer cell phagocytosis of tumor cells.
The extracellular segment of the ERMAP protein contains an immunoglobulin like V-type domain. Using this structural feature, Yuan’s group constructed an ERMAP-Fc fusion protein and searched for protein molecules binding to ERMAP on Kupffer cells using affinity purification-mass spectrometry. Galectin-9 (a glycosylated binding protein) was found to be an ERMAP binding protein. The amino acid residue N135 of mouse ERMAP and amino acid residue N132 of human ERMAP are N-linked glycosylation modified sites. Immunoprecipitation experiments showed that the mutation of ERMAP glycosylation sites or de-glycosylation treatments both reduced the binding of ERMAP to Gal-9, proving that ERMAP bound to Gal-9 in a glycosylation dependent manner. The mutation of ERMAP glycosylation sites or addition of Gal-9 neutralizing antibodies block the effect of ERMAP on promoting Kupffer cell phagocytosis. Correspondingly, the mutation of ERMAP glycosylation sites can largely block the inhibitory effect of ERMAP on tumor liver metastasis.
Considering that Gal-9 does not have a transmembrane domain and can be secreted to extracellular region, Yuan’s group further explored the membrane receptor on the surface of Kupffer cells that bound to ERMAP/Gal-9. They found that the C-type lectin family protein dectin-2 bound to ERMAP/Gal-9, and dectin-2 was specifically highly expressed in Kupffer cells. Gal-9 acted as a bridge molecule and formed a bridging complex with the ERMAP on the surface of tumor cells and the transmembrane receptor dectin-2 expressed on Kupffer cells. Both immunoprecipitation and ELISA experiments demonstrated the mutual binding of the three. Confocal microscopy showed colocalization of ERMAP, Gal-9, and dectin-2 on Kupffer cells. To verify whether dectin-2 was a key receptor mediating the action of ERMAP/Gal-9, Yuan’s group constructed dectin-2 knockout mice and found that knocking out dectin-2 largely blocked the regulatory effects of ERMAP on Kupffer cell phagocytosis and tumor liver metastasis, suggesting that ERMAP/Gal-9/dectin-2 transmits the "eat me" signal to Kupffer cells and inhibits tumor liver metastasis.
In addition, the analysis of human colorectal cancer liver metastatic tissues collected by the group and colorectal cancer liver metastasis data from the GEO database showed that the expression of ERMAP was negatively correlated with liver metastasis, but not with lung metastasis, omental metastasis, and brain metastasis. It also supported that ERMAP specific inhibited liver metastasis of tumor cells.
In summary, Yuan’s group focused on the interaction between tumor cells and the microenvironment of liver tissue during tumor liver metastasis. After nine years of research and exploration, they identified ERMAP/Gal-9/dectin-2 as a non-classical "eat me" signal independent of antibodies, which specifically promoted Kupffer cell phagocytosis of tumor cells and thereby inhibited tumor liver metastasis. Therefore, activating the ERMAP/Gal-9/dectin-2 signal axis may serve as a strategy to enhance Kupffer cell phagocytosis and anti-tumor innate immunity.
This study has received support from the Core Facility of Basic Medical Sciences, Shanghai Jiao Tong University School of Medicine and Clinical Cancer Institute, Center for Translational Medicine, Naval Medical University, as well as funding and support from the National Natural Science Foundation of China, the Shanghai Science and Technology Innovation Action Plan, and the "Double First Class" discipline construction funds of Naval Medical University.
Links: https://www.nature.com/articles/s41590-023-01634-7
The liver is a highly vascularized target organ for metastasis. Various tumor cells, such as liver cancer, colorectal cancer, pancreatic cancer, melanoma, lung cancer, etc., are prone to liver metastasis. Kupffer cells, the hepatic resident macrophages, engulf these disseminated malignant cells and as such serve as the first line of defense. However, the specific "eat me" or "don't eat me" signals that mediate Kupffer cell recognition and phagocytosis of tumor cells are still unclear. Identifying the signals regulating phagocytosis in Kupffer cells can provide a theoretical basis for developing new strategies to enhance Kupffer phagocytosis and limit tumor liver metastasis.
On October 9, 2023, Prof. Ji-hang Yuan’s group from Naval Medical University published a research paper online in Nature Immunology entitled "The ligation between ERMAP, galectin-9 and dectin-2 promotes Kupffer cell phagocytosis and anti-tumor immunity". This study found that the transmembrane protein ERMAP on tumor cells transmits the "eat me" signal to Kupffer cells through galectin-9/dectin-2, promotes Kupffer cells to engulf tumor cells, and inhibits tumor liver metastasis.
Yuan’s group conducted genome-wide CRISPR-cas9 knockout screening in an in vivo liver metastasis model, and unbiased identified genes regulating tumor liver metastasis. They found that gRNA targeting Ermap was enriched in liver metastatic tissues. Yuan’s group constructed tumor cell lines with ERMAP knocked out, knocked down, or overexpressed. In vivo liver metastasis experiments found that ERMAP inhibited tumor liver metastasis, but in vivo lung metastasis experiments found that ERMAP did not regulate tumor lung metastasis. In vitro functional experiments found that ERMAP did not regulate tumor cell proliferation, migration, and other behaviors, indicating that the specific role of ERMAP in liver metastasis was depends on the liver tissue microenvironment. Yuan’s group further found that ERMAP specifically bound to hepatic resident macrophage Kupffer cells and promoted Kupffer cell phagocytosis of tumor cells.
The extracellular segment of the ERMAP protein contains an immunoglobulin like V-type domain. Using this structural feature, Yuan’s group constructed an ERMAP-Fc fusion protein and searched for protein molecules binding to ERMAP on Kupffer cells using affinity purification-mass spectrometry. Galectin-9 (a glycosylated binding protein) was found to be an ERMAP binding protein. The amino acid residue N135 of mouse ERMAP and amino acid residue N132 of human ERMAP are N-linked glycosylation modified sites. Immunoprecipitation experiments showed that the mutation of ERMAP glycosylation sites or de-glycosylation treatments both reduced the binding of ERMAP to Gal-9, proving that ERMAP bound to Gal-9 in a glycosylation dependent manner. The mutation of ERMAP glycosylation sites or addition of Gal-9 neutralizing antibodies block the effect of ERMAP on promoting Kupffer cell phagocytosis. Correspondingly, the mutation of ERMAP glycosylation sites can largely block the inhibitory effect of ERMAP on tumor liver metastasis.
Considering that Gal-9 does not have a transmembrane domain and can be secreted to extracellular region, Yuan’s group further explored the membrane receptor on the surface of Kupffer cells that bound to ERMAP/Gal-9. They found that the C-type lectin family protein dectin-2 bound to ERMAP/Gal-9, and dectin-2 was specifically highly expressed in Kupffer cells. Gal-9 acted as a bridge molecule and formed a bridging complex with the ERMAP on the surface of tumor cells and the transmembrane receptor dectin-2 expressed on Kupffer cells. Both immunoprecipitation and ELISA experiments demonstrated the mutual binding of the three. Confocal microscopy showed colocalization of ERMAP, Gal-9, and dectin-2 on Kupffer cells. To verify whether dectin-2 was a key receptor mediating the action of ERMAP/Gal-9, Yuan’s group constructed dectin-2 knockout mice and found that knocking out dectin-2 largely blocked the regulatory effects of ERMAP on Kupffer cell phagocytosis and tumor liver metastasis, suggesting that ERMAP/Gal-9/dectin-2 transmits the "eat me" signal to Kupffer cells and inhibits tumor liver metastasis.

In addition, the analysis of human colorectal cancer liver metastatic tissues collected by the group and colorectal cancer liver metastasis data from the GEO database showed that the expression of ERMAP was negatively correlated with liver metastasis, but not with lung metastasis, omental metastasis, and brain metastasis. It also supported that ERMAP specific inhibited liver metastasis of tumor cells.
In summary, Yuan’s group focused on the interaction between tumor cells and the microenvironment of liver tissue during tumor liver metastasis. After nine years of research and exploration, they identified ERMAP/Gal-9/dectin-2 as a non-classical "eat me" signal independent of antibodies, which specifically promoted Kupffer cell phagocytosis of tumor cells and thereby inhibited tumor liver metastasis. Therefore, activating the ERMAP/Gal-9/dectin-2 signal axis may serve as a strategy to enhance Kupffer cell phagocytosis and anti-tumor innate immunity.
This study has received support from the Core Facility of Basic Medical Sciences, Shanghai Jiao Tong University School of Medicine and Clinical Cancer Institute, Center for Translational Medicine, Naval Medical University, as well as funding and support from the National Natural Science Foundation of China, the Shanghai Science and Technology Innovation Action Plan, and the "Double First Class" discipline construction funds of Naval Medical University.
Links: https://www.nature.com/articles/s41590-023-01634-7


