Prof. Min Luo’s group from Fudan University identifies a novel target on PMN-MDSCs for cancer immunotherapy
Source:Min Luo
2023-10-23
Immune checkpoint blockade has achieved considerable success in tumor treatment. However, a large proportion of patients show only minimal to no responsiveness to the treatments. The immune suppressive TME, characterized by high content of myeloid cells, represents a major barrier to effective immunotherapy.
Pathologically activated neutrophils, also known as polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), are a critical component of the TME. Distinct from classical neutrophils, PMN-MDSCs exhibit potent immune suppressive activity, playing crucial roles in tumor progression and therapy resistance. Identification of key molecule(s) involved in the recruitment and functions of PMN-MDSCs will be required to selectively target PMN-MDSCs for tumor treatment.
On September 6, 2023, the team led by Min Luo at Fudan University published a research article "CD300ld on neutrophils is required for tumour-driven immune suppression" in Nature. The study identifies a novel receptor, CD300ld, as a critical immune suppressor present on PMN-MDSCs, being required for tumor immune resistance. CD300ld is a surface marker for neutrophils/PMN-MDSCs and is required for the recruitment of PMN-MDSCs into tumors and their function to suppress T-cell activation. Blockage of CD300ld remodels the immune-suppressive TME and shows potent anti-tumor efficacy, low risk of side effects, as well as synergistic effects with immune checkpoint inhibitor, providing a promising target for cancer immunotherapy.
Neutrophils are highly abundant and heterogeneous myeloid cells, acting as the first responder to provide an innate defense against acute infection or inflammation. In cancer and some other instances of pathological chronic inflammation, aberrant granulopoiesis significantly increases neutrophil release from bone marrow. These neutrophils, particularly in late-stage cancers, are functionally perturbed, showing a high level of plasticity and promoting both direct and indirect tumorigenesis mechanisms. Such pathologically activated neutrophils exhibit potent immune suppressive activity and are functionally named PMN-MDSCs or granulocytic MDSCs (g-MDSCs), emerging as a critical component of the TME in many mouse and human tumors. Selective targeting of PMN-MDSCs represents an attractive strategy to overcome the immune suppressive nature of the TME for tumor eradication.
For this aim, the researchers of this study first performed an in-vivo CRISPR-Cas9 screening with a sgRNA library of myeloid-biased membrane proteins, and found that the CD300ld sgRNAs showed the most depletion in tumor compared to bone marrow, suggesting CD300ld as a tumor-favoring receptor. CD300ld is specifically expressed in neutrophils and is up-regulated after tumor-bearing. By generating CD300ld knockout mice, the researchers revealed that CD300ld KO inhibits tumor development in multiple tumor models and has little effects on mice development. With multiple approaches, including BM transplantation, conditional knockout and PMN-MDSCs depletion, the authors demonstrated that CD300ld regulates tumor progression via PMN-MDSCs. Therefore, CD300ld is a surface marker for neutrophils/PMN-MDSCs and a key receptor to promote tumor development.
Mechanism investigation revealed that CD300ld is required for the recruitment of PMN-MDSCs into tumors and their function to suppress T-cell activation, and the loss of CD300ld remodels the TME from immune suppressive to immune active. S100A8/A9 act as the major effector downstream of CD300ld, and STAT3 plays essential roles in CD300ld-S100A8/A9 axis to regulate the functions of PMN-MDSCs.
Moreover, CD300ld ablation at the tumor establishment phase, either by induced KO or by competitive inhibition with CD300ld extracellular domain protein, can significantly inhibit tumor development. Furthermore, CD300ld ablations exhibit synergy effect with anti-PD1. These result suggest the potential of CD300ld as a therapeutic target for cancer treatment.
The authors further investigated the roles of CD300LD in human cancers. Human CD300LD is also highly and specifically expressed in neutrophils, and is associated with poor clinical outcomes in multiple cancer types. They generated Cd300ld humanized mice and demonstrated that tumor promoting activity of Cd300ld is conserved between mouse and human.
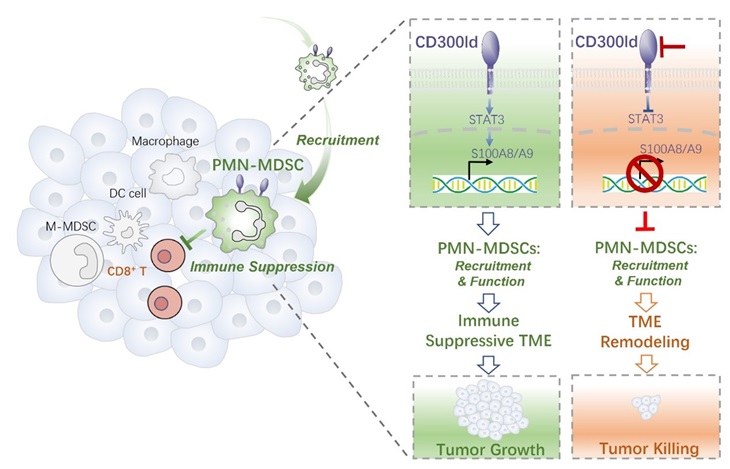
In conclusion, this study has identified CD300ld as a surface marker and a critical immune suppressor on PMN-MDSCs to promote tumor progression. CD300ld is required for the recruitment of PMN-MDSCs into tumors and their function to suppress T-cell activation. CD300ld acts via the STAT3-S100A8/A9 axis. Blockage of CD300ld remodels the immune-suppressive TME and shows potent anti-tumor efficacy, as well as synergistic effects with immune checkpoint inhibitor, providing a promising target for cancer immunotherapy.
This study was supported by the National Natural Science Foundation of China, the National Key Research and Development Program of China, Chinese Academy of Sciences, Shanghai Municipal Science and Technology, and Fudan University,
Links: https://www.nature.com/articles/s41586-023-06511-9
Pathologically activated neutrophils, also known as polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), are a critical component of the TME. Distinct from classical neutrophils, PMN-MDSCs exhibit potent immune suppressive activity, playing crucial roles in tumor progression and therapy resistance. Identification of key molecule(s) involved in the recruitment and functions of PMN-MDSCs will be required to selectively target PMN-MDSCs for tumor treatment.
On September 6, 2023, the team led by Min Luo at Fudan University published a research article "CD300ld on neutrophils is required for tumour-driven immune suppression" in Nature. The study identifies a novel receptor, CD300ld, as a critical immune suppressor present on PMN-MDSCs, being required for tumor immune resistance. CD300ld is a surface marker for neutrophils/PMN-MDSCs and is required for the recruitment of PMN-MDSCs into tumors and their function to suppress T-cell activation. Blockage of CD300ld remodels the immune-suppressive TME and shows potent anti-tumor efficacy, low risk of side effects, as well as synergistic effects with immune checkpoint inhibitor, providing a promising target for cancer immunotherapy.
Neutrophils are highly abundant and heterogeneous myeloid cells, acting as the first responder to provide an innate defense against acute infection or inflammation. In cancer and some other instances of pathological chronic inflammation, aberrant granulopoiesis significantly increases neutrophil release from bone marrow. These neutrophils, particularly in late-stage cancers, are functionally perturbed, showing a high level of plasticity and promoting both direct and indirect tumorigenesis mechanisms. Such pathologically activated neutrophils exhibit potent immune suppressive activity and are functionally named PMN-MDSCs or granulocytic MDSCs (g-MDSCs), emerging as a critical component of the TME in many mouse and human tumors. Selective targeting of PMN-MDSCs represents an attractive strategy to overcome the immune suppressive nature of the TME for tumor eradication.
For this aim, the researchers of this study first performed an in-vivo CRISPR-Cas9 screening with a sgRNA library of myeloid-biased membrane proteins, and found that the CD300ld sgRNAs showed the most depletion in tumor compared to bone marrow, suggesting CD300ld as a tumor-favoring receptor. CD300ld is specifically expressed in neutrophils and is up-regulated after tumor-bearing. By generating CD300ld knockout mice, the researchers revealed that CD300ld KO inhibits tumor development in multiple tumor models and has little effects on mice development. With multiple approaches, including BM transplantation, conditional knockout and PMN-MDSCs depletion, the authors demonstrated that CD300ld regulates tumor progression via PMN-MDSCs. Therefore, CD300ld is a surface marker for neutrophils/PMN-MDSCs and a key receptor to promote tumor development.
Mechanism investigation revealed that CD300ld is required for the recruitment of PMN-MDSCs into tumors and their function to suppress T-cell activation, and the loss of CD300ld remodels the TME from immune suppressive to immune active. S100A8/A9 act as the major effector downstream of CD300ld, and STAT3 plays essential roles in CD300ld-S100A8/A9 axis to regulate the functions of PMN-MDSCs.
Moreover, CD300ld ablation at the tumor establishment phase, either by induced KO or by competitive inhibition with CD300ld extracellular domain protein, can significantly inhibit tumor development. Furthermore, CD300ld ablations exhibit synergy effect with anti-PD1. These result suggest the potential of CD300ld as a therapeutic target for cancer treatment.
The authors further investigated the roles of CD300LD in human cancers. Human CD300LD is also highly and specifically expressed in neutrophils, and is associated with poor clinical outcomes in multiple cancer types. They generated Cd300ld humanized mice and demonstrated that tumor promoting activity of Cd300ld is conserved between mouse and human.

In conclusion, this study has identified CD300ld as a surface marker and a critical immune suppressor on PMN-MDSCs to promote tumor progression. CD300ld is required for the recruitment of PMN-MDSCs into tumors and their function to suppress T-cell activation. CD300ld acts via the STAT3-S100A8/A9 axis. Blockage of CD300ld remodels the immune-suppressive TME and shows potent anti-tumor efficacy, as well as synergistic effects with immune checkpoint inhibitor, providing a promising target for cancer immunotherapy.
This study was supported by the National Natural Science Foundation of China, the National Key Research and Development Program of China, Chinese Academy of Sciences, Shanghai Municipal Science and Technology, and Fudan University,
Links: https://www.nature.com/articles/s41586-023-06511-9


