Zhou Bo's Collaborative Team Reveals Cellular Origins of Immunoregulatory and Niche-supporting Megakaryocytes
Source:Zhou Bo
2024-03-22
In a study published in Immunity, researchers led by Prof. Zhou Bo from the Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences, together with the research group of Wang Qianfei from the Beijing Institute of Genomics, Chinese Academy of Sciences (China National Center for Bioinformation), and the research group of Cheng Tao from the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College (Hematology Research Institute) developed a novel lineage tracing system combined with single-cell sequencing to elucidate the relationship between the differentiation pathways of hematopoietic stem cells (HSCs) and the yield and functionality of megakaryocytes (MKs).
Increasing evidence suggests that HSCs (or MPPs) can directly differentiate into megakaryocytic progenitors (MkPs) without undergoing a series of restrictive progenitor (RPs) stages in the classical hematopoietic hierarchy. The question of why HSCs or MPPs have not abandoned the stepwise differentiation pathway through a series of RPs when they can directly differentiate into the megakaryocytic lineage remains unclear. Due to technological limitations, it has been challenging to compare the two differentiation pathways, and the differentiation kinetics of MkPs and MKs generated by different differentiation pathways remain unclear.
The researchers first developed a lineage tracing system capable of distinguishing between direct and stepwise hematopoietic differentiation pathways. CD48-Dre efficiently and specifically labeled RPs on the stepwise differentiation pathway, while the team constructed Rosa26loxp-STOP-loxp-rox-loxp-ZsGreen-STOP-rox-tdTomato (R26ZT1) reporter mice, using this mouse to induce the inducible tracing of CD48-Dre+ cells, and plotted the turnover curves of various hematopoietic cell lineages at different levels. The results indicated that all progenitor cells on the stepwise differentiation pathway were rapidly and completely replaced after tracing began. Using Kit-creER, which is expressed only in hematopoietic stem progenitor cells and not in mature blood cells, orthogonally recombined with CD48-Dre, the team also plotted the differentiation kinetics curves of various hematopoietic progenitor cells generating downstream mature cells on different differentiation pathways and found that both pathways contribute to megakaryocyte production in comparable quantities and with similar kinetic characteristics under steady-state conditions.
Megakaryocytes, in addition to their well-known role in producing platelets with functions such as clotting and immunity, also serve as important niches for hematopoietic stem cells (HSCs) and directly participate in immune responses. Single-cell sequencing has revealed the existence of different functional subgroups of MKs. However, the mechanisms by which megakaryocytes acquire different functions remain to be elucidated. By performing single-cell sequencing on megakaryocytes derived from two different paths distinguished by the lineage tracing system (CD48dre; R26rox-tdTomato), the research team found that niche-supporting MKs are produced by the direct differentiation pathway (Tomato-negative), while immune MKs are produced by the stepwise differentiation pathway (Tomato-positive), and both pathways jointly contribute to the production of platelet-producing MKs. Correspondingly, megakaryocytes generated through different pathways exhibited different functional activities in vitro and in vivo.
By simulating different physiological demands in vivo in tracing mice, the research team found that the contributions of the two pathways to megakaryocytes and platelets would be adjusted under different stress conditions. Under marrow-clearing pressure induced by 5-FU, the demand for hematopoietic regeneration stimulated the rapid and preferential production of megakaryocytes from the direct differentiation pathway; whereas inflammation induced by LPS preferentially stimulated the production of megakaryocytes from the stepwise differentiation pathway; and blood loss-induced platelet consumption would simultaneously accelerate the production of platelets from both pathways.
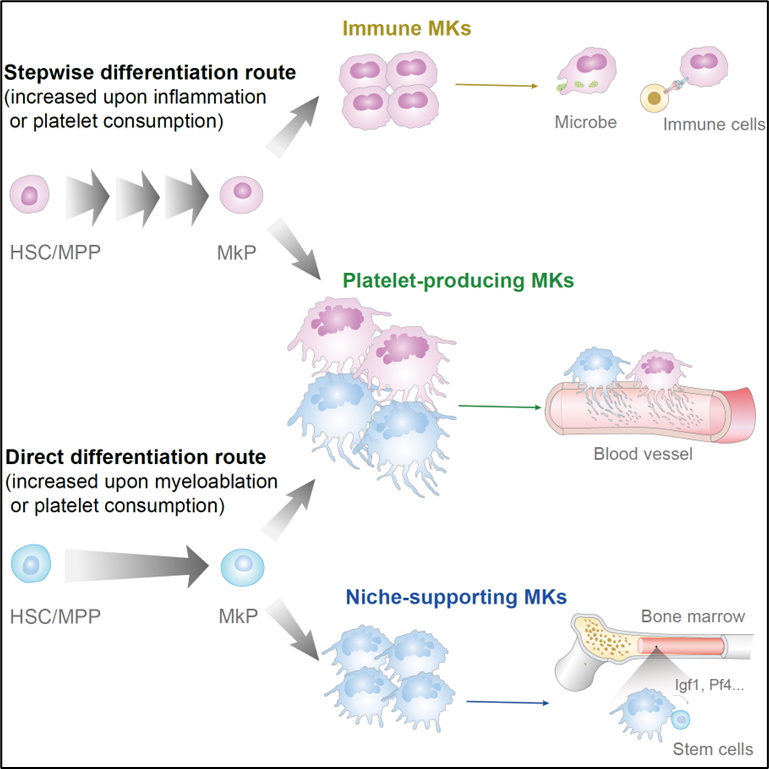
In summary, this study developed a fate tracing system that efficiently and specifically distinguishes between direct and stepwise hematopoietic differentiation pathways, providing for the first time a turnover and differentiation kinetics atlas for each lineage branch of different hematopoietic levels, filling a gap in the dynamic study of hematopoietic progenitors under steady-state conditions, and linking differentiation pathways with the functional heterogeneity of adult megakaryocytes. Moreover, the conclusion that platelet generation through different pathways is regulated by different physiological demands provides new insights into the mechanisms of platelet changes in various clinical conditions.
Links: https://www.sciencedirect.com/science/article/pii/S1074761324000827
Increasing evidence suggests that HSCs (or MPPs) can directly differentiate into megakaryocytic progenitors (MkPs) without undergoing a series of restrictive progenitor (RPs) stages in the classical hematopoietic hierarchy. The question of why HSCs or MPPs have not abandoned the stepwise differentiation pathway through a series of RPs when they can directly differentiate into the megakaryocytic lineage remains unclear. Due to technological limitations, it has been challenging to compare the two differentiation pathways, and the differentiation kinetics of MkPs and MKs generated by different differentiation pathways remain unclear.
The researchers first developed a lineage tracing system capable of distinguishing between direct and stepwise hematopoietic differentiation pathways. CD48-Dre efficiently and specifically labeled RPs on the stepwise differentiation pathway, while the team constructed Rosa26loxp-STOP-loxp-rox-loxp-ZsGreen-STOP-rox-tdTomato (R26ZT1) reporter mice, using this mouse to induce the inducible tracing of CD48-Dre+ cells, and plotted the turnover curves of various hematopoietic cell lineages at different levels. The results indicated that all progenitor cells on the stepwise differentiation pathway were rapidly and completely replaced after tracing began. Using Kit-creER, which is expressed only in hematopoietic stem progenitor cells and not in mature blood cells, orthogonally recombined with CD48-Dre, the team also plotted the differentiation kinetics curves of various hematopoietic progenitor cells generating downstream mature cells on different differentiation pathways and found that both pathways contribute to megakaryocyte production in comparable quantities and with similar kinetic characteristics under steady-state conditions.
Megakaryocytes, in addition to their well-known role in producing platelets with functions such as clotting and immunity, also serve as important niches for hematopoietic stem cells (HSCs) and directly participate in immune responses. Single-cell sequencing has revealed the existence of different functional subgroups of MKs. However, the mechanisms by which megakaryocytes acquire different functions remain to be elucidated. By performing single-cell sequencing on megakaryocytes derived from two different paths distinguished by the lineage tracing system (CD48dre; R26rox-tdTomato), the research team found that niche-supporting MKs are produced by the direct differentiation pathway (Tomato-negative), while immune MKs are produced by the stepwise differentiation pathway (Tomato-positive), and both pathways jointly contribute to the production of platelet-producing MKs. Correspondingly, megakaryocytes generated through different pathways exhibited different functional activities in vitro and in vivo.
By simulating different physiological demands in vivo in tracing mice, the research team found that the contributions of the two pathways to megakaryocytes and platelets would be adjusted under different stress conditions. Under marrow-clearing pressure induced by 5-FU, the demand for hematopoietic regeneration stimulated the rapid and preferential production of megakaryocytes from the direct differentiation pathway; whereas inflammation induced by LPS preferentially stimulated the production of megakaryocytes from the stepwise differentiation pathway; and blood loss-induced platelet consumption would simultaneously accelerate the production of platelets from both pathways.

Hematopoietic Stem Cell Differentiation Pathways Determine the Functional Diversity of Megakaryocytes/Platelets Illustration
In summary, this study developed a fate tracing system that efficiently and specifically distinguishes between direct and stepwise hematopoietic differentiation pathways, providing for the first time a turnover and differentiation kinetics atlas for each lineage branch of different hematopoietic levels, filling a gap in the dynamic study of hematopoietic progenitors under steady-state conditions, and linking differentiation pathways with the functional heterogeneity of adult megakaryocytes. Moreover, the conclusion that platelet generation through different pathways is regulated by different physiological demands provides new insights into the mechanisms of platelet changes in various clinical conditions.
Links: https://www.sciencedirect.com/science/article/pii/S1074761324000827


