Prof. Ping Gao and Prof. Huafeng Zhang’ s group reveal a novel mechanism of carnosine in regulating intracellular pH homeostasis and lysosome-dependent tumor immunoevasion
Source:Ronghui Yan
2024-03-28
Despite a reliance on glycolysis for energy metabolism, most cancer cells exhibit an inverted pH gradient, with a lower extra-cellular pH (6.7–7.1) than intracellular pH (7.2–7.4). Previous research on the regulation of intracellular pH in cancer cells has focused on the activity of ion pumps and transporters at the cell membrane. However, there is a gap in our understanding of how the H+ generated by glycolysis in the cytoplasm migrates to membrane transporters and is ultimately exported into the extracellular space. Complicating this question, the cytoplasm contains an abundance of proteins with H+ titratable groups to which free H+ ions readily anchor, resulting in low cytoplasmic mobility of H+ ions. In addition, although emerging evidence suggests that intracellular pH may have abundant roles in regulating biological processes, our knowledge about whether and how it affects tumor progression, and tumor immunity in particular, is limited.
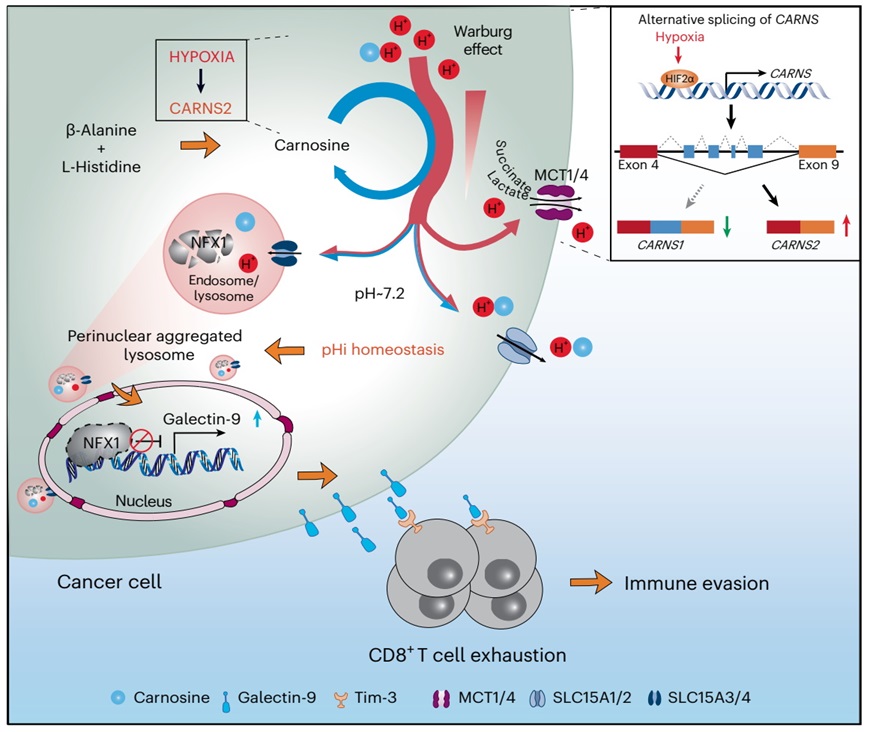
On January 04, 2024, a research article entitled “Carnosine regulation of intracellular pH homeostasis promotes lysosome-dependent tumor immunoevasion” was published online in Nature Immunology. This study was led by Professor Ping Gao from Southern Medical University/Guangdong Provincial People's Hospital and Professor Huafeng Zhang from University of Science and Technology of China and found that, a previously unrecognized isoform of carnosine synthase, CARNS2, is identified to promote carnosine synthesis under hypoxia. Carnosine maintains pHi homeostasis by functioning as a mobile proton carrier to accelerate cytosolic H+ mobility and release, which in turn controls lysosomal subcellular distribution, acidification and activity. Furthermore, by maintaining lysosomal activity, carnosine facilitates nuclear transcription factor, X-box binding 1 (NFX1) degradation, triggering Galectin-9 and T cell-mediated immune escape and tumorigenesis.
Previous research has suggested that intrinsic buffering agents can act as mobile proton carriers that help H+ to diffuse freely throughout the cytosolic environment. To determine whether and which of these molecules function in tumor cells, the research team quantified the levels of these metabolites in HepG2 cells cultured in either normoxic or hypoxic conditions using liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS), and noticed that carnosine, a metabolite that may have key roles in maintaining homeostasis of intracellular pH based on its pKa characteristics, accumulated under hypoxia. By comparing the size of H+-related currents in cells treated with exogenous carnosine and untreated cells, and measuring intracellular pH when manipulating intracellular carnosine levels, the research team confirmed that carnosine can accelerate cytosolic H+ mobility, release and ultimately maintain pH homeostasis. Immunoblotting assays showed increased expression of a previously unrecognized isoform of carnosine synthase (CARNS2) in hypoxia, and identified CARNS2 as the main carnosine synthase in hepatocellular carcinoma (HCC) cells. These data suggest that the observed accumulation of carnosine in HCC cells under hypoxia is due to the activity of CARNS2.
Further investigations revealed that in hypoxia, carnosine or CARNS2 controls lysosomal subcellular distribution, acidification and activity. Using lysosomal proteomics screening and RNA-sequencing analysis, they discovered that the transcriptional repressor NFX1 is markedly degraded in lysosomes under hypoxia, leading to increased galectin-9 expression. Data from in vitro culture in conditional medium and in vivo mouse models demonstrated that a CARNS2-NFX1-galectin-9 regulatory axis promotes immune evasion by inhibiting the abundance and functions of CD8+ T cells, which is highly relevant to liver cancer progression. Finally, data from immunoblotting and immunohistochemistry studies in clinical liver cancer samples also suggest that the CARNS2-NFX1-galectin-9 axis is highly relevant to the development of human HCC.
In summary, this study uncovers a previously unrecognized mechanism through which tumor cells resist intracellular overacidification to maintain pH homeostasis. It also reveals a link between carnosine-regulated pHi homeostasis and tumor immune escape, and provide a conceptual basis for development of combination therapies that include pHi intervention, lysosome inhibition, and immune checkpoint blockade to improve the effectiveness of HCC treatments.
Dr. Ronghui Yan and Dr. Pinggen Zhang at University of Science and Technology of China (USTC) are the co-first authors of this paper. In addition, Prof. Chunlei Cang and Prof. Weidong Jia at USTC have provided firm support for the research. This work was supported in part by National Natural Science Foundation of China, National Key R&D Program of China, the Chinese Academy of Sciences, the Global Select Project of the Institute of Health and Medicine, Hefei Comprehensive National Science Center.
Links: https://www.nature.com/articles/s41590-023-01719-3
On January 04, 2024, a research article entitled “Carnosine regulation of intracellular pH homeostasis promotes lysosome-dependent tumor immunoevasion” was published online in Nature Immunology. This study was led by Professor Ping Gao from Southern Medical University/Guangdong Provincial People's Hospital and Professor Huafeng Zhang from University of Science and Technology of China and found that, a previously unrecognized isoform of carnosine synthase, CARNS2, is identified to promote carnosine synthesis under hypoxia. Carnosine maintains pHi homeostasis by functioning as a mobile proton carrier to accelerate cytosolic H+ mobility and release, which in turn controls lysosomal subcellular distribution, acidification and activity. Furthermore, by maintaining lysosomal activity, carnosine facilitates nuclear transcription factor, X-box binding 1 (NFX1) degradation, triggering Galectin-9 and T cell-mediated immune escape and tumorigenesis.
Previous research has suggested that intrinsic buffering agents can act as mobile proton carriers that help H+ to diffuse freely throughout the cytosolic environment. To determine whether and which of these molecules function in tumor cells, the research team quantified the levels of these metabolites in HepG2 cells cultured in either normoxic or hypoxic conditions using liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS), and noticed that carnosine, a metabolite that may have key roles in maintaining homeostasis of intracellular pH based on its pKa characteristics, accumulated under hypoxia. By comparing the size of H+-related currents in cells treated with exogenous carnosine and untreated cells, and measuring intracellular pH when manipulating intracellular carnosine levels, the research team confirmed that carnosine can accelerate cytosolic H+ mobility, release and ultimately maintain pH homeostasis. Immunoblotting assays showed increased expression of a previously unrecognized isoform of carnosine synthase (CARNS2) in hypoxia, and identified CARNS2 as the main carnosine synthase in hepatocellular carcinoma (HCC) cells. These data suggest that the observed accumulation of carnosine in HCC cells under hypoxia is due to the activity of CARNS2.
Further investigations revealed that in hypoxia, carnosine or CARNS2 controls lysosomal subcellular distribution, acidification and activity. Using lysosomal proteomics screening and RNA-sequencing analysis, they discovered that the transcriptional repressor NFX1 is markedly degraded in lysosomes under hypoxia, leading to increased galectin-9 expression. Data from in vitro culture in conditional medium and in vivo mouse models demonstrated that a CARNS2-NFX1-galectin-9 regulatory axis promotes immune evasion by inhibiting the abundance and functions of CD8+ T cells, which is highly relevant to liver cancer progression. Finally, data from immunoblotting and immunohistochemistry studies in clinical liver cancer samples also suggest that the CARNS2-NFX1-galectin-9 axis is highly relevant to the development of human HCC.
In summary, this study uncovers a previously unrecognized mechanism through which tumor cells resist intracellular overacidification to maintain pH homeostasis. It also reveals a link between carnosine-regulated pHi homeostasis and tumor immune escape, and provide a conceptual basis for development of combination therapies that include pHi intervention, lysosome inhibition, and immune checkpoint blockade to improve the effectiveness of HCC treatments.

Dr. Ronghui Yan and Dr. Pinggen Zhang at University of Science and Technology of China (USTC) are the co-first authors of this paper. In addition, Prof. Chunlei Cang and Prof. Weidong Jia at USTC have provided firm support for the research. This work was supported in part by National Natural Science Foundation of China, National Key R&D Program of China, the Chinese Academy of Sciences, the Global Select Project of the Institute of Health and Medicine, Hefei Comprehensive National Science Center.
Links: https://www.nature.com/articles/s41590-023-01719-3


