Prof. Rongbin Zhou’s group summarize the research progress of DAMPs and sterile inflammation in the 30th anniversary special issue of Immunity
Source:Rongbin Zhou
2024-04-25
Damage-associated molecular patterns (DAMPs) are endogenous signaling molecules produced in cellular damage or stress, and they can activate the innate immune system. Although these endogenous molecules do not induce inflammatory responses under steady-state condition, they may undergo changes in distribution, physical or chemical property, or concentration upon cellular damage or stress, and then they become DAMPs that can be sensed by innate immune receptors to induce inflammatory response. DAMPs and DAMP-mediated inflammatory responses play key roles in a variety of major chronic diseases. Therefore, a deep understanding of DAMPs and sterile inflammation will help to reveal the mechanism of immune responses and develop new therapy strategy of inflammatory diseases.
Professor Rongbin Zhou's group from the University of Science and Technology of China was invited to write a review for the 30th anniversary special issue of Immunity. On April 10, 2024, this review entitled " DAMPs and DAMP-sensing receptors in inflammation and diseases " was published online on April 10, 2024. They summarize the conversion of homeostatic molecules into DAMPs; the diverse nature and classification, cellular origin, and sensing of DAMPs; and their role in inflammation and related diseases. They also discuss the clinical strategies to treat DAMP-associated diseases via targeting DAMP-sensing receptors.
1. The routes for endogenous molecule conversion to DAMPs
Under steady-state conditions, endogenous molecules, as self-components of the host, are usually immune privileged and do not induce inflammatory responses. However, when cells encounter damage or stress, some endogenous molecules undergo changes of their location, concentration or property to convert into pro-inflammatory DAMPs. For example, the displacement from a tolerated position to a site where they are no longer tolerated is the primary way by which endogenous molecules are converted into DAMPs. The changes of properties, including physical properties and chemical properties, are also able to convert endogenous molecules into DAMPs. The pro-inflammatory activity of some DAMPs depends on their concentration. At low concentration, they will not induce inflammatory responses, but at high concentration, they are regarded as danger signals.
2. The diverse nature and classification of DAMPs
According to molecular characteristics, DAMPs can be divided into five categories: nucleic acids, proteins, ions, glycans, and metabolites. They can be recognized by DAMP-sensing receptors in the cytoplasm or on the cell surface, thereby activating inflammatory responses. On the one hand, the diversity of DAMP molecular characteristics ensures immediate response to alarm signals; on the other hand, the diversity of DAMPs will also lead to the involvement of multiple DAMPs in a single inflammatory disease, making the pathological mechanism extremely complex.
3. The cellular origin of DAMPs
DAMPs play key roles in both inflammatory response and tissue repair, so the production of DAMPs needs to be strictly controlled. Recent studies have found that cellular events such as cell death, organelle damage or stress, and metabolic dysregulation are the main sources of DAMPs. For example, leakage of intracellular substances caused by cell death such as necrosis, pyroptosis, and ferroptosis can expose intracellular substance, allowing them to interact with receptors and induce inflammatory responses. Damaged and stressed organelles such as ER, ribosome, and mitochondria can also produce DAMPs to promote inflammatory response. Metabolic dysregulation can lead to changes in the concentration of various endogenous molecules, which might induce inflammatory responses. Collectively, cell death, organelle damage, stress, and metabolic dysregulation are the main causes of the conversion of endogenous molecules into DAMPs in cells. It is helpful to further identify the key DAMPs and reveal their role in roles and mechanisms in inflammation and diseases.
4. DAMP sensing and their role in inflammation in diseases
DAMPs activate inflammatory signaling pathways by acting on DAMP-sensing receptors. Therefore, DAMP-sensing receptors play important roles in DAMP-associated inflammation and diseases. Initially, some pattern recognition receptors (PRRs) that used to be considered as pathogen sensors have been found to recognize endogenous danger molecules and mediate inflammatory responses. The activation of these PRRs triggers the activation of downstream signaling pathways to produce inflammatory cytokines, such as IL-6, IL-1β, and I-IFN, leading to development of neurodegenerative diseases, metabolic diseases, tumors and other diseases. In recent years, some new receptors, such as GPCRs, have also been found to be involved in DAMP sensing.
5. The strategies targeting DAMP-sensing receptors in diseases
Since DAMP-sensing receptors are involved in the progression of many major diseases, targeting DAMP-sensing receptors may provide new strategies to treat these diseases. In pre-clinical studies, the effects of some antagonists of DAMP-sensing receptors have been proved in many mouse models, such as Alzheimer's disease, type 2 diabetes, and gout. Although targeting DAMP-sensing receptors have received encouraging results in animal models, only a few of them have been tested in clinical trials. Therefore, there is an urgent need to develop new drugs targeting DAMP-sensing receptors to prove that targeting DAMP-related inflammation may be an effective immunotherapy for these diseases.
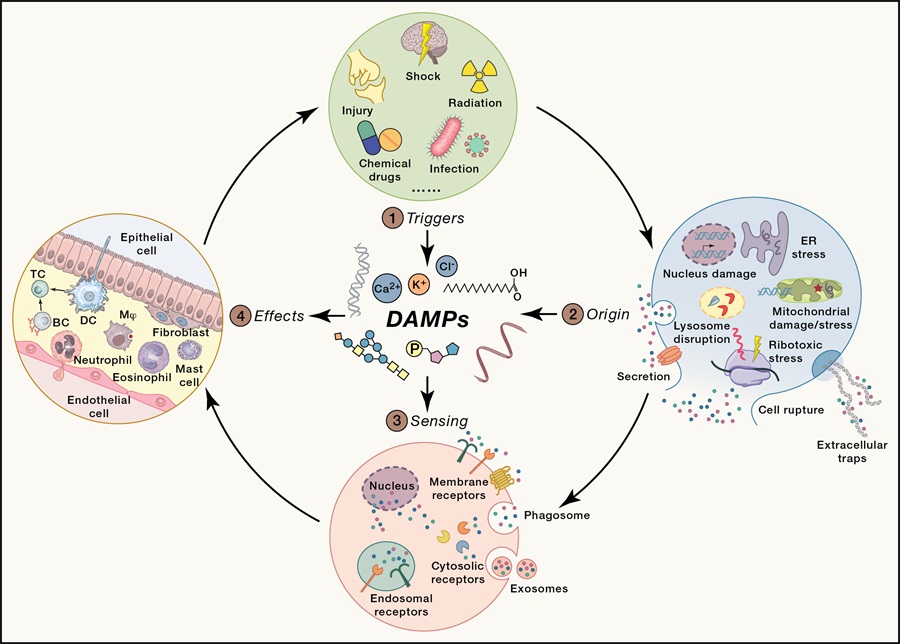
This review summarizes three main pathways for the conversion of endogenous molecules into DAMPs, systematically introduces the classification, cell origin and sensing mechanisms of DAMPs, and provides an outlook on the urgent problems that need to be solved, which shows directions of further studies in this field.
Links: https://www.cell.com/immunity/fulltext/S1074-7613(24)00098-0
Professor Rongbin Zhou's group from the University of Science and Technology of China was invited to write a review for the 30th anniversary special issue of Immunity. On April 10, 2024, this review entitled " DAMPs and DAMP-sensing receptors in inflammation and diseases " was published online on April 10, 2024. They summarize the conversion of homeostatic molecules into DAMPs; the diverse nature and classification, cellular origin, and sensing of DAMPs; and their role in inflammation and related diseases. They also discuss the clinical strategies to treat DAMP-associated diseases via targeting DAMP-sensing receptors.
1. The routes for endogenous molecule conversion to DAMPs
Under steady-state conditions, endogenous molecules, as self-components of the host, are usually immune privileged and do not induce inflammatory responses. However, when cells encounter damage or stress, some endogenous molecules undergo changes of their location, concentration or property to convert into pro-inflammatory DAMPs. For example, the displacement from a tolerated position to a site where they are no longer tolerated is the primary way by which endogenous molecules are converted into DAMPs. The changes of properties, including physical properties and chemical properties, are also able to convert endogenous molecules into DAMPs. The pro-inflammatory activity of some DAMPs depends on their concentration. At low concentration, they will not induce inflammatory responses, but at high concentration, they are regarded as danger signals.
2. The diverse nature and classification of DAMPs
According to molecular characteristics, DAMPs can be divided into five categories: nucleic acids, proteins, ions, glycans, and metabolites. They can be recognized by DAMP-sensing receptors in the cytoplasm or on the cell surface, thereby activating inflammatory responses. On the one hand, the diversity of DAMP molecular characteristics ensures immediate response to alarm signals; on the other hand, the diversity of DAMPs will also lead to the involvement of multiple DAMPs in a single inflammatory disease, making the pathological mechanism extremely complex.
3. The cellular origin of DAMPs
DAMPs play key roles in both inflammatory response and tissue repair, so the production of DAMPs needs to be strictly controlled. Recent studies have found that cellular events such as cell death, organelle damage or stress, and metabolic dysregulation are the main sources of DAMPs. For example, leakage of intracellular substances caused by cell death such as necrosis, pyroptosis, and ferroptosis can expose intracellular substance, allowing them to interact with receptors and induce inflammatory responses. Damaged and stressed organelles such as ER, ribosome, and mitochondria can also produce DAMPs to promote inflammatory response. Metabolic dysregulation can lead to changes in the concentration of various endogenous molecules, which might induce inflammatory responses. Collectively, cell death, organelle damage, stress, and metabolic dysregulation are the main causes of the conversion of endogenous molecules into DAMPs in cells. It is helpful to further identify the key DAMPs and reveal their role in roles and mechanisms in inflammation and diseases.
4. DAMP sensing and their role in inflammation in diseases
DAMPs activate inflammatory signaling pathways by acting on DAMP-sensing receptors. Therefore, DAMP-sensing receptors play important roles in DAMP-associated inflammation and diseases. Initially, some pattern recognition receptors (PRRs) that used to be considered as pathogen sensors have been found to recognize endogenous danger molecules and mediate inflammatory responses. The activation of these PRRs triggers the activation of downstream signaling pathways to produce inflammatory cytokines, such as IL-6, IL-1β, and I-IFN, leading to development of neurodegenerative diseases, metabolic diseases, tumors and other diseases. In recent years, some new receptors, such as GPCRs, have also been found to be involved in DAMP sensing.
5. The strategies targeting DAMP-sensing receptors in diseases
Since DAMP-sensing receptors are involved in the progression of many major diseases, targeting DAMP-sensing receptors may provide new strategies to treat these diseases. In pre-clinical studies, the effects of some antagonists of DAMP-sensing receptors have been proved in many mouse models, such as Alzheimer's disease, type 2 diabetes, and gout. Although targeting DAMP-sensing receptors have received encouraging results in animal models, only a few of them have been tested in clinical trials. Therefore, there is an urgent need to develop new drugs targeting DAMP-sensing receptors to prove that targeting DAMP-related inflammation may be an effective immunotherapy for these diseases.

This review summarizes three main pathways for the conversion of endogenous molecules into DAMPs, systematically introduces the classification, cell origin and sensing mechanisms of DAMPs, and provides an outlook on the urgent problems that need to be solved, which shows directions of further studies in this field.
Links: https://www.cell.com/immunity/fulltext/S1074-7613(24)00098-0


