Prof. Zhuowei Hu’s group reveals a crucial role of the Ubiquitin-Editing Enzyme A20 in alveolar macrophage during pulmonary fibrosis
Source:Zhuowei Hu
2019-09-23
On August 27, 2019, Prof. Zhuowei Hu’s group of Institute of Materia Medica, Chinese Academy of Medical Sciences published a research article in Immunity entitled “Targeting the Transcriptional Factor C/EBPβ Degradation Reduces Lung Fibrosis by Restoring Activity of the Ubiquitin-Editing Enzyme A20 in Macrophages”. This study revealed that glycogen synthase kinase-3β(GSK-3β) interacts with and phosphorylates A20 to impede its activity, causing progressive accumulation of the transcription factor C/EBPβ in alveolar macrophages (AMs), which directs the profibrotic phenotype of macrophages promoting pulmonary fibrosis (PF) development. This finding provides a new drug target for treating PF and fibroproliferative lung diseases.
In recent years, Zhuowei Hu's group has been working on the molecular and cellular biological mechanisms of pulmonary fibrosis from the perspective of immunobiology, and on this basis, developed drugs that can prevent and treat PF. Macrophages, as innate immune cells, secrete a variety of inflammatory mediators involved in the pathogenesis of PF. Ubiquitin-editing enzyme A20 participates in the process of inflammatory diseases by regulating a variety of cellular biological functions. However, whether A20 regulates macrophage function and participates in the pathogenesis of PF remains unclear. In the present study, the group found that overexpression of A20 in macrophages significantly reduced bleomycin-induced PF, whereas deletion of A20 in macrophages caused much more serious fibrotic changes. Then the team performed single-cell sequencing to analyze the effect of overexpression of A20 on macrophage phenotype. The results showed that overexpression of A20 significantly inhibited the profibrotic phenotype of AMs. Importantly, the authors found that the enzymatic activity of A20 in AMs from PF patients and mice was significantly suppressed, which causes and maintains the profibrotic phenotype of alveolar macrophages.
Mechanistically, the transcription factor C/EBPβ in macrophages was a ubiquitination substrate of A20. A20 promoted the degradation of C/EBPβ by reducing its K63-linked ubiquitination and enhancing the K48-linked ubiquitination. Inhibition of A20 activity in PF led to the accumulation of C/EBPβ in AMs, which promoted the conversion of AMs to a profibrotic phenotype. The team also found that the expression of GSK-3β was elevated in AMs from PF patients and mice, which interacted with and phosphorylated A20 to suppress its enzymatic activity. These results suggest that GSK3β-A20-C/EBPβ axis is an important cause of alveolar macrophage activation in PF. And the interaction of GSK3β and A20 may be a potential target for the treatment of pulmonary fibrosis. On this basis, the researchers screened a small-molecule peptide probe that could disturb the GSK3β/A20 interaction by analyzing the amino acid sequence of their interaction domain. The group then confirmed that the interfering peptide showed potent therapeutic efficacy against lung fibrosis in different models.
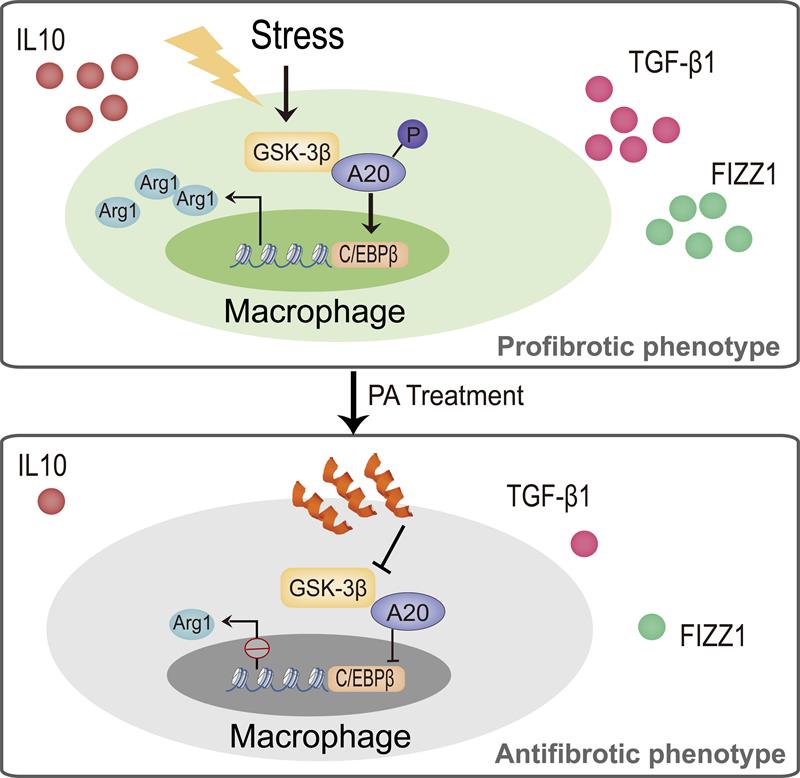
This study explains the molecular mechanism of the pathogenesis of PF from a new perspective. Researchers from Prof. Zhuowei Hu’s group not only identified the GSK3β/A20 interaction as a new target for PF treatment, but also discovered a therapeutic peptide for PF patients through targeting this interaction, demonstrating the potential of molecular mechanism research to transform into clinical applications.
Prof. Zhuowei Hu is the corresponding author of this article. Dr. Shanshan Liu and Dr. Xiaoxi Lv are the co-first authors. Ph.D. students Chang Liu, Jie Qi, Yunxuan Li also contributed to this study. This study was supported by National Key R&D Program of China, National Natural Science Foundation of China, Chinese Academy of Medical Sciences (CAMS) Central Public-interest Scientific Institution Basal Research Fund, and CAMS Innovation Found for Medical Sciences.
Links: https://www.cell.com/immunity/fulltext/S1074-7613(19)30284-5
In recent years, Zhuowei Hu's group has been working on the molecular and cellular biological mechanisms of pulmonary fibrosis from the perspective of immunobiology, and on this basis, developed drugs that can prevent and treat PF. Macrophages, as innate immune cells, secrete a variety of inflammatory mediators involved in the pathogenesis of PF. Ubiquitin-editing enzyme A20 participates in the process of inflammatory diseases by regulating a variety of cellular biological functions. However, whether A20 regulates macrophage function and participates in the pathogenesis of PF remains unclear. In the present study, the group found that overexpression of A20 in macrophages significantly reduced bleomycin-induced PF, whereas deletion of A20 in macrophages caused much more serious fibrotic changes. Then the team performed single-cell sequencing to analyze the effect of overexpression of A20 on macrophage phenotype. The results showed that overexpression of A20 significantly inhibited the profibrotic phenotype of AMs. Importantly, the authors found that the enzymatic activity of A20 in AMs from PF patients and mice was significantly suppressed, which causes and maintains the profibrotic phenotype of alveolar macrophages.
Mechanistically, the transcription factor C/EBPβ in macrophages was a ubiquitination substrate of A20. A20 promoted the degradation of C/EBPβ by reducing its K63-linked ubiquitination and enhancing the K48-linked ubiquitination. Inhibition of A20 activity in PF led to the accumulation of C/EBPβ in AMs, which promoted the conversion of AMs to a profibrotic phenotype. The team also found that the expression of GSK-3β was elevated in AMs from PF patients and mice, which interacted with and phosphorylated A20 to suppress its enzymatic activity. These results suggest that GSK3β-A20-C/EBPβ axis is an important cause of alveolar macrophage activation in PF. And the interaction of GSK3β and A20 may be a potential target for the treatment of pulmonary fibrosis. On this basis, the researchers screened a small-molecule peptide probe that could disturb the GSK3β/A20 interaction by analyzing the amino acid sequence of their interaction domain. The group then confirmed that the interfering peptide showed potent therapeutic efficacy against lung fibrosis in different models.

This study explains the molecular mechanism of the pathogenesis of PF from a new perspective. Researchers from Prof. Zhuowei Hu’s group not only identified the GSK3β/A20 interaction as a new target for PF treatment, but also discovered a therapeutic peptide for PF patients through targeting this interaction, demonstrating the potential of molecular mechanism research to transform into clinical applications.
Prof. Zhuowei Hu is the corresponding author of this article. Dr. Shanshan Liu and Dr. Xiaoxi Lv are the co-first authors. Ph.D. students Chang Liu, Jie Qi, Yunxuan Li also contributed to this study. This study was supported by National Key R&D Program of China, National Natural Science Foundation of China, Chinese Academy of Medical Sciences (CAMS) Central Public-interest Scientific Institution Basal Research Fund, and CAMS Innovation Found for Medical Sciences.
Links: https://www.cell.com/immunity/fulltext/S1074-7613(19)30284-5


