Xuetao Cao’s group reports new lncRNA Sros1 and its regulatory role in host cell resistance against intracellular bacterial infection
Source:Henan Xu
2019-12-23
Prof. Xuetao Cao (Peking Union Medical College & Chinese Academy of Medical Sciences) and his group published a research article in Nature Immunology about the inhibitory mechanism of lncRNA Sros1 for interferon-γ function. (Henan Xu, Yan Jiang, Xiaoqing Xu, Xiaoping Su, Yang Liu, Yuanwu Ma, Yong Zhao, Zhongyang Shen, Bo Huang & Xuetao Cao. Inducible degradation of lncRNA Sros1 promotes IFN-γ-mediated activation of innate immune responses by stabilizing Stat1 mRNA. 2019 Dec; 20(12):1621-1630).
Innate immune responses protect the host from pathogen infection and exert evolutionary pressure on pathogens to evolve strategies to attenuate these host responses and ensure their survival and replication. These evolving pressures have led to intricate mechanisms of innate immune homeostasis across diverse host–pathogen interactions that are not comprehensively understood.
Supported by grants from the National Natural Science Foundation of China, Chinese Academy of Medical Sciences' Innovation Fund for Medical Sciences and the National Key Research and Development Program of China, Postdoctor Henan Xu and her colleagues from Prof. Xuetao Cao’s group screened and found microRNA-1(miR-1)promoted IFN-γ-mediated clearance of intracellular bacteria in macrophages. Using miRIP-seq, they demonstrated that miR-1 indirectly stabilized the Stat1 messenger RNA through the degradation of the cytoplasmic long noncoding RNA Sros1(suppressive non-coding RNA of STAT1). Inducible degradation or genetic loss of Sros1 led to enhanced IFN-γ-dependent activation of the innate immune response by destabilizing Stat1 mRNA. In vivo infection mouse model demonstrated thatdeficiency of Sros1 protects mice against L. monocytogenes infection by enhancing Stat1 expression and IFN-γ signaling. Mechanistically, Sros1 blocked the binding of Stat1 mRNA to the RBP CAPRIN1, which stabilized the Stat1 mRNA and, consequently, promoted IFN-γ–STAT1-mediated innate immunity.
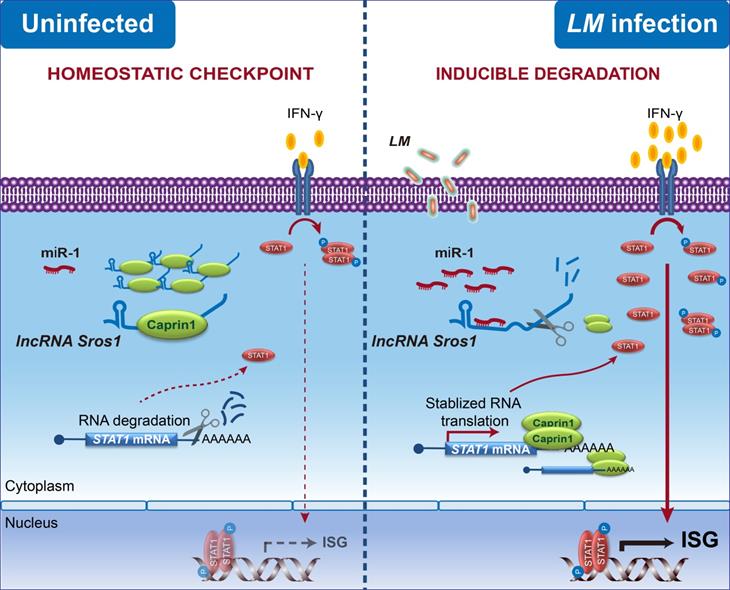
These observations shed light on the complex RNA–RNA regulatory networks involved in cytokine-initiated innate responses in host–pathogen interactions. These findings suggest that it may be worthwhile conducting future research on lncRNA-mediated RNA metabolism of transcription factors, RNA–RNA interactions and post-transcriptional regulatory networks relevant to cytokine biology and pathology. These results also highlight dynamic functional interactions among miRNAs, lncRNAs, mRNAs and RBPs in immune cells, underscoring the finely tuned regulatory mechanisms that contribute to innate immune homeostasis physiologically and the susceptibility to infection pathologically.
Links: https://www.nature.com/articles/s41590-019-0542-7
Innate immune responses protect the host from pathogen infection and exert evolutionary pressure on pathogens to evolve strategies to attenuate these host responses and ensure their survival and replication. These evolving pressures have led to intricate mechanisms of innate immune homeostasis across diverse host–pathogen interactions that are not comprehensively understood.
Supported by grants from the National Natural Science Foundation of China, Chinese Academy of Medical Sciences' Innovation Fund for Medical Sciences and the National Key Research and Development Program of China, Postdoctor Henan Xu and her colleagues from Prof. Xuetao Cao’s group screened and found microRNA-1(miR-1)promoted IFN-γ-mediated clearance of intracellular bacteria in macrophages. Using miRIP-seq, they demonstrated that miR-1 indirectly stabilized the Stat1 messenger RNA through the degradation of the cytoplasmic long noncoding RNA Sros1(suppressive non-coding RNA of STAT1). Inducible degradation or genetic loss of Sros1 led to enhanced IFN-γ-dependent activation of the innate immune response by destabilizing Stat1 mRNA. In vivo infection mouse model demonstrated thatdeficiency of Sros1 protects mice against L. monocytogenes infection by enhancing Stat1 expression and IFN-γ signaling. Mechanistically, Sros1 blocked the binding of Stat1 mRNA to the RBP CAPRIN1, which stabilized the Stat1 mRNA and, consequently, promoted IFN-γ–STAT1-mediated innate immunity.

These observations shed light on the complex RNA–RNA regulatory networks involved in cytokine-initiated innate responses in host–pathogen interactions. These findings suggest that it may be worthwhile conducting future research on lncRNA-mediated RNA metabolism of transcription factors, RNA–RNA interactions and post-transcriptional regulatory networks relevant to cytokine biology and pathology. These results also highlight dynamic functional interactions among miRNAs, lncRNAs, mRNAs and RBPs in immune cells, underscoring the finely tuned regulatory mechanisms that contribute to innate immune homeostasis physiologically and the susceptibility to infection pathologically.
Links: https://www.nature.com/articles/s41590-019-0542-7


