Prof. Zhengfan Jiang’s group reveals that sulfated glycosaminoglycans are coligands for STING activation
Source:Zhengfan Jiang
2021-05-07
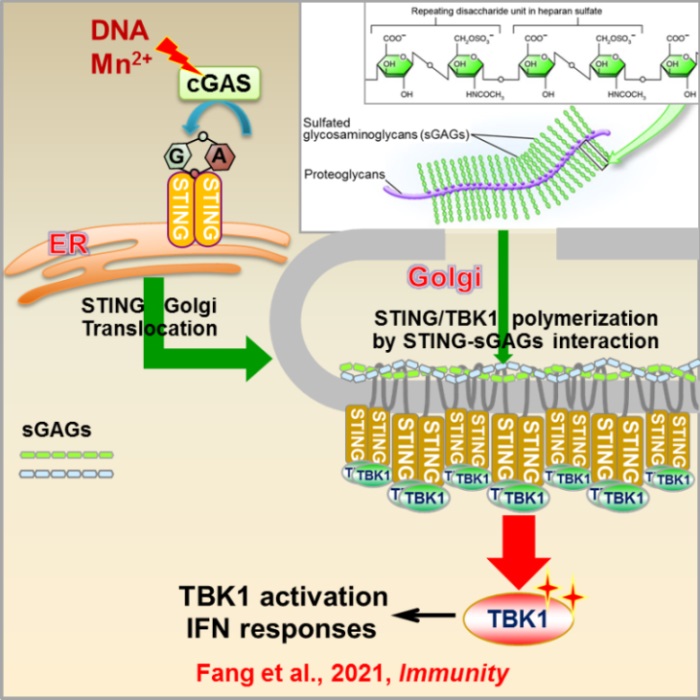
On April 14, the Cell Press journal IMMUNITY published the research article “Golgi apparatus-synthesized sulfated glycosaminoglycans mediate polymerization and activation of the cGAMP sensor STING”. This study led by Jiang’s group at Peking university, demonstrated that Golgi apparatus-synthesized sulfated glycosaminoglycans (sGAGs) interact with STING to initiate its oligomerization at the Golgi apparatus, thus identifying sGAGs as necessary coligands for STING activation and downstream IFN signaling.
The cGAS-STING pathway is responsible for detection of abnormally located cytoplasmic DNA that serves as a danger signal of pathogen invasion or tissue damage. The cytosolic DNA sensor cGAS is activated by DNA binding or Mn2+ to produce the second messenger 2’3’-cGAMP, which binds to and activates the endoplasmic reticulum (ER) membrane adaptor protein STING (also known as MITA, ERIS, or MPYS). When activated, STING translocates from the ER membrane to the Golgi apparatus and is polymerized to recruit and activate the kinase TBK1 and, subsequently, the transcription factor IRF3, ultimately leading to production of various cytokines, including type I IFNs. Although the structures of the cGAMP-bound STING tetramer and cGAMP-bound STING dimer with TBK1 reveal the requirements of STING polymerization for efficient signal activation, it remains unknown how STING polymers are formed in vivo. Moreover, recent studies have demonstrated that Golgi apparatus-accumulated STING undergoes autoactivation, indicating that STING could be activated without cGAMP binding. The factors in the Golgi apparatus that drive STING activation remain unknown.
Glycosaminoglycans (GAGs) are a class of long unbranched polymers of amino and uronic sugars. Based on repeating disaccharides, GAGs are categorized into four groups: heparin (Hp), Hp sulfate (HS), chondroitin sulfate (CS) and dermatan sulfate (DS), Keratan sulfate (KS), and Hyaluronic acid (HA). Except HA, which is synthesized at the plasma membrane, other GAGs are synthesized in the Golgi apparatus and are covalently attached to core proteins with specified linkage oligosaccharides to form proteoglycans (PGs). For GAGs synthesized in the Golgi apparatus, the resulting polysaccharides are subsequently modified by epimerization and sulfation to produce sulfated GAGs (sGAGs). sGAGs are localized widely at the extracellular matrix, cell surface, and intracellular vesicles, and have multiple biological functions, mainly by interacting with proteins. Negatively charged sulfated groups in sGAGs provide a multivalent landing plug for proteins through electrostatic interactions. These sulfate groups interact with various proteins by binding and neutralizing positively charged or polar residues of targeted proteins and facilitating their polymerization or binding to other proteins. Although extracellular sGAGs have been implicated in many inflammatory and infectious diseases through modulation of immune response to different cytokines, such as interleukins (ILs) and type I interferons (IFNs), the role of intracellular sGAGs is unknown.
In this study, using a genome-wide CRISPR-Cas9-mediated screen and biochemical analysis, Jiang’s group discovered that sGAGs in the Golgi apparatus or sGAGs-containing vesicles directly drove STING polymerization and activation. STING bound sGAGs through its luminal, positively charged, polar residues and the strength of the STING-sGAG interaction determined the level of STING activation. Purified or chemically synthesized sGAGs directly induced STING polymerization and activation of the kinase TBK1 in vitro. The chain length and O-linked sulfation of sGAGs directly affected the level of STING polymerization and, therefore, its activation. Importantly, sGAG-driven STING polymerization and activation is evolutionally conserved. This study not only identifies sGAGs as co-ligands for STING activation, resolves a long-standing puzzle in the field regarding STING activation upon translocation to Golgi, but also provide a potential mechanisms of STING regulation through targeting STING-sGAG interaction in autoinflammatory diseases and antitumor therapies.
Prof. Zhengfan Jiang from the School of Life science at Peking University is the corresponding author of this work. Dr. Run Fang and Qifei Jiang in the laboratory are co-first authors. The work was funded in part by National Natural Science Foundation of China, the National Key R&D Program of China, Key Laboratory of Cell Proliferation and Differentiation of the Ministry of Education and the Peking-Tsinghua Center for Life Sciences.
Links: https://www.cell.com/immunity/fulltext/S1074-7613(21)00123-0
The cGAS-STING pathway is responsible for detection of abnormally located cytoplasmic DNA that serves as a danger signal of pathogen invasion or tissue damage. The cytosolic DNA sensor cGAS is activated by DNA binding or Mn2+ to produce the second messenger 2’3’-cGAMP, which binds to and activates the endoplasmic reticulum (ER) membrane adaptor protein STING (also known as MITA, ERIS, or MPYS). When activated, STING translocates from the ER membrane to the Golgi apparatus and is polymerized to recruit and activate the kinase TBK1 and, subsequently, the transcription factor IRF3, ultimately leading to production of various cytokines, including type I IFNs. Although the structures of the cGAMP-bound STING tetramer and cGAMP-bound STING dimer with TBK1 reveal the requirements of STING polymerization for efficient signal activation, it remains unknown how STING polymers are formed in vivo. Moreover, recent studies have demonstrated that Golgi apparatus-accumulated STING undergoes autoactivation, indicating that STING could be activated without cGAMP binding. The factors in the Golgi apparatus that drive STING activation remain unknown.
Glycosaminoglycans (GAGs) are a class of long unbranched polymers of amino and uronic sugars. Based on repeating disaccharides, GAGs are categorized into four groups: heparin (Hp), Hp sulfate (HS), chondroitin sulfate (CS) and dermatan sulfate (DS), Keratan sulfate (KS), and Hyaluronic acid (HA). Except HA, which is synthesized at the plasma membrane, other GAGs are synthesized in the Golgi apparatus and are covalently attached to core proteins with specified linkage oligosaccharides to form proteoglycans (PGs). For GAGs synthesized in the Golgi apparatus, the resulting polysaccharides are subsequently modified by epimerization and sulfation to produce sulfated GAGs (sGAGs). sGAGs are localized widely at the extracellular matrix, cell surface, and intracellular vesicles, and have multiple biological functions, mainly by interacting with proteins. Negatively charged sulfated groups in sGAGs provide a multivalent landing plug for proteins through electrostatic interactions. These sulfate groups interact with various proteins by binding and neutralizing positively charged or polar residues of targeted proteins and facilitating their polymerization or binding to other proteins. Although extracellular sGAGs have been implicated in many inflammatory and infectious diseases through modulation of immune response to different cytokines, such as interleukins (ILs) and type I interferons (IFNs), the role of intracellular sGAGs is unknown.
In this study, using a genome-wide CRISPR-Cas9-mediated screen and biochemical analysis, Jiang’s group discovered that sGAGs in the Golgi apparatus or sGAGs-containing vesicles directly drove STING polymerization and activation. STING bound sGAGs through its luminal, positively charged, polar residues and the strength of the STING-sGAG interaction determined the level of STING activation. Purified or chemically synthesized sGAGs directly induced STING polymerization and activation of the kinase TBK1 in vitro. The chain length and O-linked sulfation of sGAGs directly affected the level of STING polymerization and, therefore, its activation. Importantly, sGAG-driven STING polymerization and activation is evolutionally conserved. This study not only identifies sGAGs as co-ligands for STING activation, resolves a long-standing puzzle in the field regarding STING activation upon translocation to Golgi, but also provide a potential mechanisms of STING regulation through targeting STING-sGAG interaction in autoinflammatory diseases and antitumor therapies.

Sulfated glycosaminoglycans in Golgi apparatus mediate STING-TBK1 polymerization and activation
Prof. Zhengfan Jiang from the School of Life science at Peking University is the corresponding author of this work. Dr. Run Fang and Qifei Jiang in the laboratory are co-first authors. The work was funded in part by National Natural Science Foundation of China, the National Key R&D Program of China, Key Laboratory of Cell Proliferation and Differentiation of the Ministry of Education and the Peking-Tsinghua Center for Life Sciences.
Links: https://www.cell.com/immunity/fulltext/S1074-7613(21)00123-0


