Prof. Bing Su's group reveals a new type of intestinal stromal cells and its role in repairing damaged intestine
Source:Bing Su
2021-05-24
On April 22, 2021, Nature published a research article entitled "MAP3k2-regulated intestinal stromal cells define a distinct stem cell niche" by Prof. Bing Su's group from Shanghai Institute of Immunology, Shanghai Jiao Tong University School of Medicine. This study identified a novel type of intestinal stromal cells named MRISC, localized underneath the intestinal stem cells. By using single cell RNA-sequencing, ATAC-sequencing, intestinal organoid co-culture system, and in situ injection of intestinal stromal cells, this work systematically revealed the involvement of MRISC in the intestinal epithelial tissue repair by specifically regulating the R-spondin1-Wnt signal axis in the microenvironment of intestinal stem cells during the process of intestinal inflammation (Fig), and provides new insights into intestinal tissue regeneration and clinical treatment for inflammatory bowel disease.
Tissue inflammation and damage can rapidly stimulate the proliferation of intestinal stem cells for tissue repair and regeneration, but the key regulatory signal for intestinal stem cells in inflammation is still largely unclear. Intestinal stromal cells are important components of intestinal stem cell niche. They interact with intestinal epithelial cells, immune cells, and neurons to regulate intestinal homeostasis and the function of epithelial stem cells. With rapid development of single-cell RNA-sequencing technology, researchers have revealed the intestinal mesenchymal stromal cells as a large group of under-appreciated, phenotypically and functionally heterogeneous cells. Identification of novel intestinal stromal cell subsets, characterization of their upstream stimulatory signals, and elucidation of the downstream transcriptional and epigenetic regulatory pathways is fundamental and crucial for studying stromal cells.
This study first found that the evolutionarily conserved Ser/Thr protein kinase MAP3K2 played a key role in protecting the mice from DSS induced colitis by maintaining the number of Lgr5+ stem cells in the damaged intestine. MAP3K2 up-regulated the expression of R-spondin1 following DSS induced damage. It was also shown that intestinal stromal cells were the key source of R-spondin1. To identify the surface markers for MRISC, researchers identified CD81, CD34, and CD138 by single cell RNA-sequencing and FACS sorting, and confirmed the role of MRISC for intestinal stem cells. They also developed a novel Rspo1-tdTomato reporter mouse line to show that MRISC location in intestine.
To characterize the molecular mechanism of MAP3K2 in regulating the expression of R-spondin1 in MRISC and explore its epigenetic characteristics, researchers used ATAC-seq to show that MRISC were very different from other stromal cells. The researchers also suggested the human counterpart of mouse MRISC and speculated their role in intestinal inflammation.
Through the identification of MRISC and other intestinal stromal cell subsets, we realize that stromal cells may contain functionally distinct subsets, very much like CD4+ T cells, which contain Th1, Th2, Th17 or Treg cells. Future in-depth study of stromal cell subsets will shed light for our understanding of the function of stromal cells in vivo.
Dr. Wu Ningbo and Dr. Sun Hongxiang contributed equally to this study. The corresponding author for this study is Prof. Su Bing from Shanghai Institute of Immunology, Shanghai Jiaotong University School of Medicine. The work was done in collaboration with Prof. Florent Ginhoux and Lai Guan Ng from Singapore Immunology Network (SIgN), Prof. Zhou Bin and Yi Arial Zeng from Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Prof. Richard Flavell from Yale University, Prof. Zou Duowu from Ruijin Hospital, Prof. Liu Zhiduo, Chen Lei, Ye Youqiong and Li Hua-bing from Shanghai Institute of immunology, and Prof. Cheng Jinke from Shanghai Jiaotong University School of Medicine. This work was supported in part by grants from the National Natural Science Foundation of China, Shanghai Science and Technology Commission, and The State Key Laboratory of Oncogenes and Related Genes, as well as the core facility from Shanghai Institute of Immunology.
Professor Su Bing is actively looking for talented Postdoctoral Fellows to join our wonderful team to continue the exciting investigation on stromal cells. Contact information: bingsu@sjtu.edu.cn
Links: https://www.nature.com/articles/s41586-021-03283-y

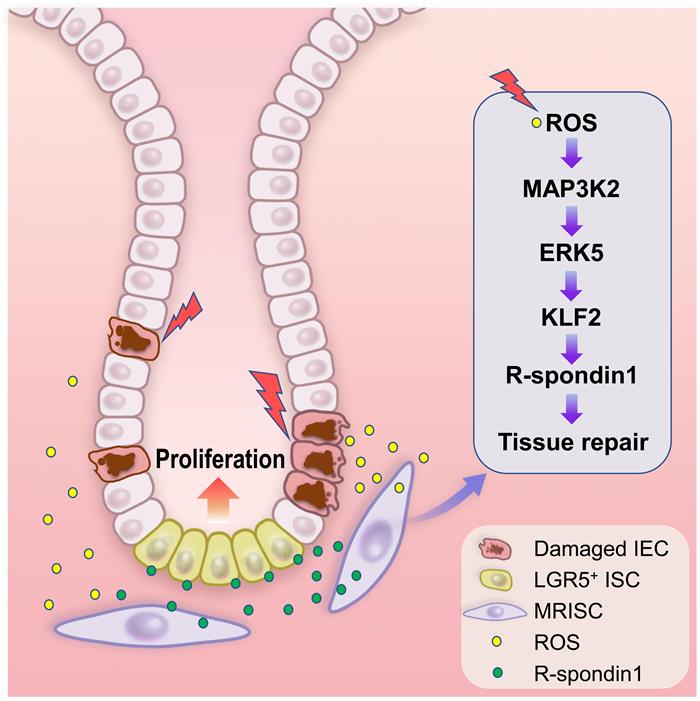
Figure. A graphic model illustrating the function and regulation of MRISC in promoting the repair of damaged intestinal epithelium
MRISC sense ROS (reactive oxygen species) signal following intestine injury to activate the MAP3K2-ERK5-KLF2 signal axis, leading to the up-regulation of R-spondin1 expression, which in turn augments the Wnt signal in intestinal stem cells to promote the repair of intestinal epithelium.
Tissue inflammation and damage can rapidly stimulate the proliferation of intestinal stem cells for tissue repair and regeneration, but the key regulatory signal for intestinal stem cells in inflammation is still largely unclear. Intestinal stromal cells are important components of intestinal stem cell niche. They interact with intestinal epithelial cells, immune cells, and neurons to regulate intestinal homeostasis and the function of epithelial stem cells. With rapid development of single-cell RNA-sequencing technology, researchers have revealed the intestinal mesenchymal stromal cells as a large group of under-appreciated, phenotypically and functionally heterogeneous cells. Identification of novel intestinal stromal cell subsets, characterization of their upstream stimulatory signals, and elucidation of the downstream transcriptional and epigenetic regulatory pathways is fundamental and crucial for studying stromal cells.
This study first found that the evolutionarily conserved Ser/Thr protein kinase MAP3K2 played a key role in protecting the mice from DSS induced colitis by maintaining the number of Lgr5+ stem cells in the damaged intestine. MAP3K2 up-regulated the expression of R-spondin1 following DSS induced damage. It was also shown that intestinal stromal cells were the key source of R-spondin1. To identify the surface markers for MRISC, researchers identified CD81, CD34, and CD138 by single cell RNA-sequencing and FACS sorting, and confirmed the role of MRISC for intestinal stem cells. They also developed a novel Rspo1-tdTomato reporter mouse line to show that MRISC location in intestine.
To characterize the molecular mechanism of MAP3K2 in regulating the expression of R-spondin1 in MRISC and explore its epigenetic characteristics, researchers used ATAC-seq to show that MRISC were very different from other stromal cells. The researchers also suggested the human counterpart of mouse MRISC and speculated their role in intestinal inflammation.
Through the identification of MRISC and other intestinal stromal cell subsets, we realize that stromal cells may contain functionally distinct subsets, very much like CD4+ T cells, which contain Th1, Th2, Th17 or Treg cells. Future in-depth study of stromal cell subsets will shed light for our understanding of the function of stromal cells in vivo.
Dr. Wu Ningbo and Dr. Sun Hongxiang contributed equally to this study. The corresponding author for this study is Prof. Su Bing from Shanghai Institute of Immunology, Shanghai Jiaotong University School of Medicine. The work was done in collaboration with Prof. Florent Ginhoux and Lai Guan Ng from Singapore Immunology Network (SIgN), Prof. Zhou Bin and Yi Arial Zeng from Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Prof. Richard Flavell from Yale University, Prof. Zou Duowu from Ruijin Hospital, Prof. Liu Zhiduo, Chen Lei, Ye Youqiong and Li Hua-bing from Shanghai Institute of immunology, and Prof. Cheng Jinke from Shanghai Jiaotong University School of Medicine. This work was supported in part by grants from the National Natural Science Foundation of China, Shanghai Science and Technology Commission, and The State Key Laboratory of Oncogenes and Related Genes, as well as the core facility from Shanghai Institute of Immunology.
Professor Su Bing is actively looking for talented Postdoctoral Fellows to join our wonderful team to continue the exciting investigation on stromal cells. Contact information: bingsu@sjtu.edu.cn
Links: https://www.nature.com/articles/s41586-021-03283-y


