Prof. Beicheng Sun’s and Anning Lin’s team unveils a novel inflammation-cancer transformation mechanism in Hepatocellular carcinoma
Source:Beicheng Sun
2021-07-02
On May 25th, 2021, the Cell Press journal IMMUNITY published the research article “The Zinc Finger Protein Miz1 Suppresses Liver Tumorigenesis by Restricting Hepatocyte-driven Macrophage Activation and Inflammation” by Beicheng Sun’s team from the Affiliated Drum Tower Hospital of Nanjing University Medical School and Anning Lin’s team from the Institute of Modern Biology, Nanjing University. Their findings demonstrate that hepatocyte Miz1 competes with RelA for binding to MTDH and also inhibits MTDH phosphorylation by IKK in a transcription-independent manner, thereby inhibiting NF-κB activation and suppressing the HCC development.
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide, typically caused by exposure to carcinogens, infection of hepatitis viruses (HBV or HCV), fatty liver diseases and environmental pollutants, followed by fibrosis and cirrhosis that induce liver damage. Chronic inflammation has a causative relationship with HCC development by contributing to liver tumor initiation and progression. The major risk factors, such as chronic HBV and HCV infection, alcohol, carcinogen-DNA damage and obesity, contribute to the inflammatory tumor microenvironment (TME) in HCC. However, the contribution by tumor hepatocytes to the inflammation is not well understood. Necrotic hepatocyte death promotes the inflammation, which in turn stimulates compensatory proliferation of survived tumor cells in chemical HCC mouse models. By contrast, apoptotic transformed hepatocytes inhibit the inflammation in inflammation-associated HCC mouse model. Hepatocyte NF-κB is typically activated in a substantial fraction of HCC patients, except in some cases where NF-κB is inactivated by infected viruses. It is not known whether live hepatocytes contribute to the inflammation and if so, what the mechanism is.
The findings of Beicheng Sun’s and Anning Lin’s team revealed that either gain or loss of NF-κB activation in hepatocyte can drive the formation of TME, thus promoting the initiation and development of HCC. By utilizing single cell RNA-sequencing in DEN/CCl4- and STZ/HFD-induced HCC, they in-depth analyzed the crosstalk between hepatocyte and macrophages in HCC. Hepatocyte-specific Miz1 Loss generates a distinct sub-group of hepatocytes highly expressing pro-Inflammatory cytokines and chemokines (Tnf, Il1b, Il6 and Ccl4 etc.), which skews the polarization of liver tumor-infiltrating macrophages toward the pro-inflammatory phenotype, thereby augmenting the liver inflammation and accelerating the HCC development. Mechanistically, TNFα-induced cytosolic Miz1 degradation releases MTDH for its subsequent phosphorylation by IKK, thereby leading to the activation of NF-κB and the expression of downstream target genes independently of its transcriptional activation. Pro-inflammatory cytokine-producing hepatocytes are increased, whereas Miz1 expression is decreased along with augmented phosphorylation of hepatocyte RelA and MTDH in a significant fraction of HCC patients with disease recurrence and poor prognosis. The expression of Miz1 is also an independent prognostic predictor for both overall survival (OS) and disease-free survival (DFS).
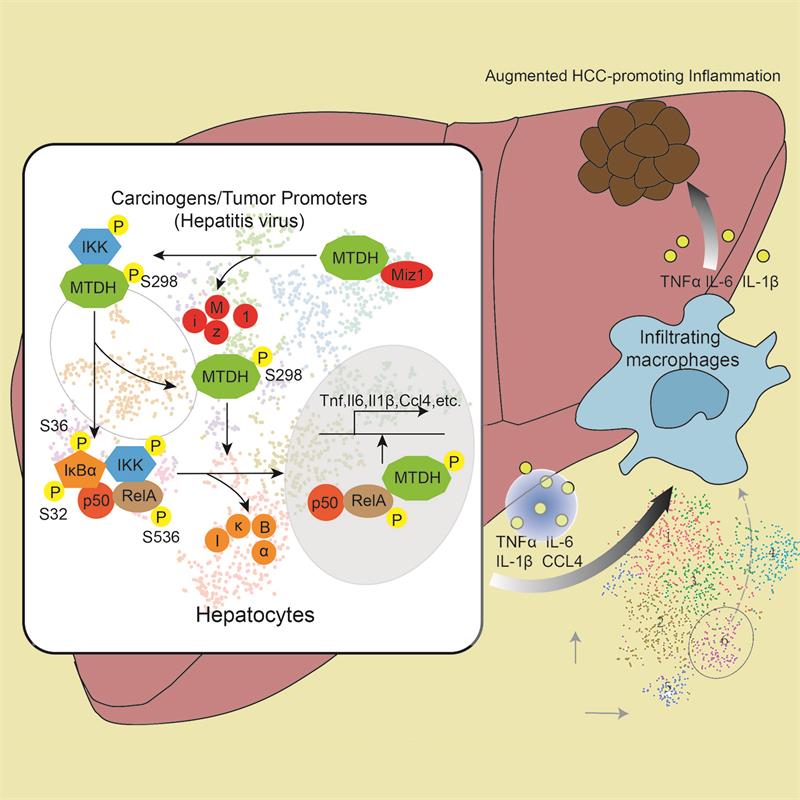
This study unveiled a novel inflammation-cancer transformation mechanism in HCC that Miz1 loss driven NF-κB activation in live hepatocyte contributes to the inflammation in TME independently of its transcriptional activation, which provides a new insight for the individualized therapy in HCC patients.
Prof. Beicheng Sun from the Affiliated Drum Tower Hospital of Nanjing University Medical School and Prof. Anning Lin from the Institute of Modern Biology are co- corresponding authors. Dr. Wenjie Zhang, Dr. Guangyan Zhangyuan and Dr. Fei Wang are co-first authors. This work was funded by grants from the National Natural Science Foundation of China, National Key Research and Development Program of China, the State Key Program of National Natural Science Foundation and National Institutes of Health grants.
Links: https://www.cell.com/immunity/fulltext/S1074-7613(21)00189-8
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide, typically caused by exposure to carcinogens, infection of hepatitis viruses (HBV or HCV), fatty liver diseases and environmental pollutants, followed by fibrosis and cirrhosis that induce liver damage. Chronic inflammation has a causative relationship with HCC development by contributing to liver tumor initiation and progression. The major risk factors, such as chronic HBV and HCV infection, alcohol, carcinogen-DNA damage and obesity, contribute to the inflammatory tumor microenvironment (TME) in HCC. However, the contribution by tumor hepatocytes to the inflammation is not well understood. Necrotic hepatocyte death promotes the inflammation, which in turn stimulates compensatory proliferation of survived tumor cells in chemical HCC mouse models. By contrast, apoptotic transformed hepatocytes inhibit the inflammation in inflammation-associated HCC mouse model. Hepatocyte NF-κB is typically activated in a substantial fraction of HCC patients, except in some cases where NF-κB is inactivated by infected viruses. It is not known whether live hepatocytes contribute to the inflammation and if so, what the mechanism is.
The findings of Beicheng Sun’s and Anning Lin’s team revealed that either gain or loss of NF-κB activation in hepatocyte can drive the formation of TME, thus promoting the initiation and development of HCC. By utilizing single cell RNA-sequencing in DEN/CCl4- and STZ/HFD-induced HCC, they in-depth analyzed the crosstalk between hepatocyte and macrophages in HCC. Hepatocyte-specific Miz1 Loss generates a distinct sub-group of hepatocytes highly expressing pro-Inflammatory cytokines and chemokines (Tnf, Il1b, Il6 and Ccl4 etc.), which skews the polarization of liver tumor-infiltrating macrophages toward the pro-inflammatory phenotype, thereby augmenting the liver inflammation and accelerating the HCC development. Mechanistically, TNFα-induced cytosolic Miz1 degradation releases MTDH for its subsequent phosphorylation by IKK, thereby leading to the activation of NF-κB and the expression of downstream target genes independently of its transcriptional activation. Pro-inflammatory cytokine-producing hepatocytes are increased, whereas Miz1 expression is decreased along with augmented phosphorylation of hepatocyte RelA and MTDH in a significant fraction of HCC patients with disease recurrence and poor prognosis. The expression of Miz1 is also an independent prognostic predictor for both overall survival (OS) and disease-free survival (DFS).

This study unveiled a novel inflammation-cancer transformation mechanism in HCC that Miz1 loss driven NF-κB activation in live hepatocyte contributes to the inflammation in TME independently of its transcriptional activation, which provides a new insight for the individualized therapy in HCC patients.
Prof. Beicheng Sun from the Affiliated Drum Tower Hospital of Nanjing University Medical School and Prof. Anning Lin from the Institute of Modern Biology are co- corresponding authors. Dr. Wenjie Zhang, Dr. Guangyan Zhangyuan and Dr. Fei Wang are co-first authors. This work was funded by grants from the National Natural Science Foundation of China, National Key Research and Development Program of China, the State Key Program of National Natural Science Foundation and National Institutes of Health grants.
Links: https://www.cell.com/immunity/fulltext/S1074-7613(21)00189-8


