Dr. Bin Li’s group reveals mechanisms underlying adipose Treg cell development and function
Source:Bin Li
2021-09-14
Dr. Bin Li’s group from Shanghai Institute of Immunology published a research paper in Nature Immunology on August 15th, 2021, entitled “Insulin signaling establishes a developmental trajectory of adipose regulatory T cells”. This study reveals novel mechanisms underlying adipose Treg cell development and function.
Adipose regulatory T (Treg) cells critically maintain immune homeostasis and metabolic health. In adipose tissue, environmental cues, including antigens, IL-33, IFN-α, insulin and androgen, fine-tune Treg cells to express unique gene modules, leading to tissue adaptation and functional diversity of tissue-resident Tregs. In this study, using single-cell ATAC-sequencing and paired single-cell RNA and T cell receptor (TCR) sequencing to map mouse adipose Treg cells, researchers identified CD73hiST2lo and CD73loST2hi subsets with distinct clonal expansion patterns. Analysis of TCR-sharing data implied a state transition between CD73hiST2lo and CD73loST2hi subsets.
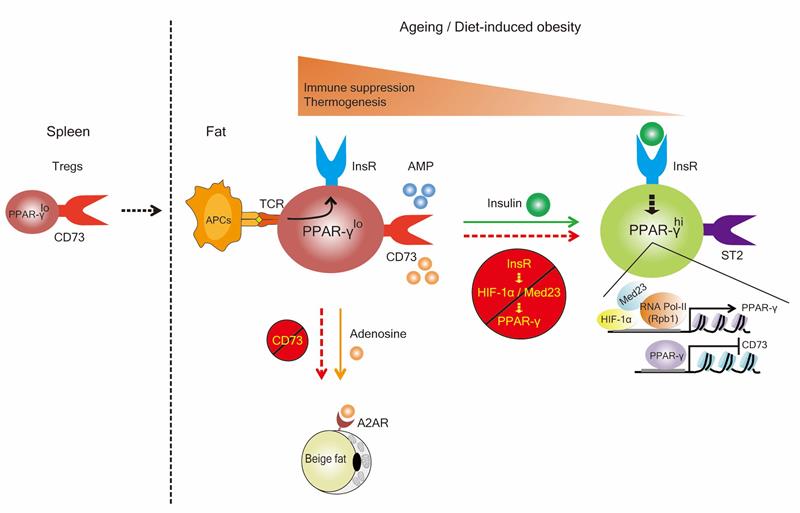
Mechanistically, researchers further revealed that insulin signaling occurs through a HIF-1α–Med23–PPAR-γ axis to drive the transition of CD73hiST2lo into a CD73loST2hi adipose Treg cell subset. Treg cells deficient in insulin receptor, HIF-1α or Med23 have decreased PPAR-γ expression that in turn promotes accumulation of CD73hiST2lo adipose Treg cells and physiological adenosine production to activate beige fat biogenesis. By contrast, ST2hi Treg subset produced higher levels of soluble isoform of ST2 (sST2), which functioned as a decoy receptor to diminish IL-33-mediated induction of beige fat biogenesis. This study therefore unveiled a developmental trajectory of adipose Treg cells and its dependence on insulin signaling (as shown in Figure), revealed dynamics and functional diversity of tissue-resident Treg subsets and reconciled previous discrepant findings by Diane Mathis and Ye Zheng groups in aged and obese fat. The knowledge gained from these efforts may facilitate the development of new therapeutics with which to treat type II diabetes.
Dr. Yangyang Li from Shanghai Institute of Immunology and Dr. Ying Lu from School of Medicine at Shanghai Jiao Tong University are co-first authors. Dr. Bin Li from Shanghai Institute of Immunology, Dr. Xuemei Tong from School of Medicine at Shanghai Jiao Tong University and Dr. Gang Wang from Fudan University are co-corresponding authors. This work was funded by grants from the National Natural Science Foundation of China, National Key R&D Program of China and Program of Shanghai Academic Research Leader.
Links: https://doi.org/10.1038/s41590-021-01010-3
Adipose regulatory T (Treg) cells critically maintain immune homeostasis and metabolic health. In adipose tissue, environmental cues, including antigens, IL-33, IFN-α, insulin and androgen, fine-tune Treg cells to express unique gene modules, leading to tissue adaptation and functional diversity of tissue-resident Tregs. In this study, using single-cell ATAC-sequencing and paired single-cell RNA and T cell receptor (TCR) sequencing to map mouse adipose Treg cells, researchers identified CD73hiST2lo and CD73loST2hi subsets with distinct clonal expansion patterns. Analysis of TCR-sharing data implied a state transition between CD73hiST2lo and CD73loST2hi subsets.
Mechanistically, researchers further revealed that insulin signaling occurs through a HIF-1α–Med23–PPAR-γ axis to drive the transition of CD73hiST2lo into a CD73loST2hi adipose Treg cell subset. Treg cells deficient in insulin receptor, HIF-1α or Med23 have decreased PPAR-γ expression that in turn promotes accumulation of CD73hiST2lo adipose Treg cells and physiological adenosine production to activate beige fat biogenesis. By contrast, ST2hi Treg subset produced higher levels of soluble isoform of ST2 (sST2), which functioned as a decoy receptor to diminish IL-33-mediated induction of beige fat biogenesis. This study therefore unveiled a developmental trajectory of adipose Treg cells and its dependence on insulin signaling (as shown in Figure), revealed dynamics and functional diversity of tissue-resident Treg subsets and reconciled previous discrepant findings by Diane Mathis and Ye Zheng groups in aged and obese fat. The knowledge gained from these efforts may facilitate the development of new therapeutics with which to treat type II diabetes.

Figure. Mechanistic models underlying adipose Treg cell development
Dr. Yangyang Li from Shanghai Institute of Immunology and Dr. Ying Lu from School of Medicine at Shanghai Jiao Tong University are co-first authors. Dr. Bin Li from Shanghai Institute of Immunology, Dr. Xuemei Tong from School of Medicine at Shanghai Jiao Tong University and Dr. Gang Wang from Fudan University are co-corresponding authors. This work was funded by grants from the National Natural Science Foundation of China, National Key R&D Program of China and Program of Shanghai Academic Research Leader.
Links: https://doi.org/10.1038/s41590-021-01010-3


