Study at Westlake University reveals early developing B cells undergo tolerance selection at the brain borders
Source:Heping Xu
2021-12-24
Researchers at Westlake University reveal that developing B cells in the meninges undergo a central nervous system (CNS) antigen-dependent checkpoint to prevent autoreactive B cells from accumulating at the CNS borders.
B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. B cells are at the center of the adaptive humoral immune system: they recognize a wide variety of soluble materials (usually protein antigens) of pathogens or vaccines via immunoglobulins anchored on their surface and differentiate into antibody-producing cells, called plasma cells, capable of secreting immunoglobulins (typically known as antibodies) to neutralize invasive pathogens.
However, many newly generated B cells could recognize antigens of our own body and thereby potentially secret self-reactive and destructive antibodies. These self-reactive B cells are usually cleared through multiple quality checkpoints, including a powerful step named as central tolerance selection, to avoid autoimmune disorders like lupus, multiple sclerosis and many others. Since the discovery of B cells in the 1960s, it’s believed that early B cell development and central tolerance selection is solely present in the bone marrow of mammals during adulthood.
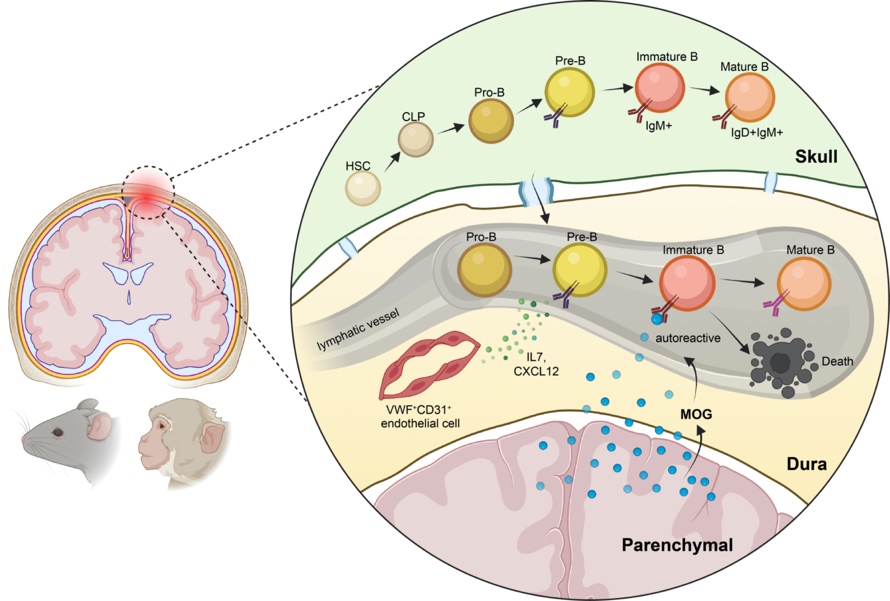
In a new Immunity study, researchers at Westlake University, including co-first authors Yan Wang and Dianyu Chen, two graduate students, and Di Xu, a Research Assistant, uncovers a consecutive trajectory of B cell development in the meninges (the tissues that surround the brain and spinal cord) of mice and rhesus macaques. Using various experimental animal models, they demonstrate that meningeal developing B cells were continuously replenished from hematopoietic stem cell (HSC)-derived progenitors via a circulation-independent route. Furthermore, the CNS-self-reactive developing B cells are eliminated specifically from the meninges via the in situ tolerance checkpoint.
“There has been a knowledge gap on how exactly B cell tolerance selection against highly tissue-specific antigens is achieved if the event only occurs in the bone marrow” said Heping Xu PhD, an immunologist and Assistant Professor in the School of Life Sciences, Westlake University. Xu is the co-senior author on the paper. “We noticed that B cells are present in the meninges according to recent publications, but whether and how potentially self-reactive B cells respond to CNS-specific antigens in the meninges is unknown. What we’ve found is that there’s a new and conserved in situ negative selection mechanism to ensure a locally non-self-reactive immune repertoire.”
“Understanding how immune repertoire is shaped at the brain borders under homeostasis and diseases is essential to dissect the basic mechanisms underlying neuro-immune interactions” said Danyang He PhD, an Assistant Professor of Neuroimmunology in the School of Life Sciences, Westlake University. He co-advised the project and is the co-senior author on the paper. “The discovery of meningeal B cell development call for a reassessment of the current understanding of B cell development and tolerance checkpoints, and shed light on the etiology of autoimmune encephalitis.”
Funding for this project provided in part by the National Key R&D program of China (grants 2020YFA0804200 and 2019YFA0802900), National Natural Science Foundation of China (grants U20A20346, 31970842 and 32070953), Westlake Laboratory of Life Sciences and Biomedicine and the Education Foundation of Westlake University.
Links: https://doi.org/10.1016/j.immuni.2021.09.016

Graph abstract of meningeal B cell development and tolerance selection
B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. B cells are at the center of the adaptive humoral immune system: they recognize a wide variety of soluble materials (usually protein antigens) of pathogens or vaccines via immunoglobulins anchored on their surface and differentiate into antibody-producing cells, called plasma cells, capable of secreting immunoglobulins (typically known as antibodies) to neutralize invasive pathogens.
However, many newly generated B cells could recognize antigens of our own body and thereby potentially secret self-reactive and destructive antibodies. These self-reactive B cells are usually cleared through multiple quality checkpoints, including a powerful step named as central tolerance selection, to avoid autoimmune disorders like lupus, multiple sclerosis and many others. Since the discovery of B cells in the 1960s, it’s believed that early B cell development and central tolerance selection is solely present in the bone marrow of mammals during adulthood.
In a new Immunity study, researchers at Westlake University, including co-first authors Yan Wang and Dianyu Chen, two graduate students, and Di Xu, a Research Assistant, uncovers a consecutive trajectory of B cell development in the meninges (the tissues that surround the brain and spinal cord) of mice and rhesus macaques. Using various experimental animal models, they demonstrate that meningeal developing B cells were continuously replenished from hematopoietic stem cell (HSC)-derived progenitors via a circulation-independent route. Furthermore, the CNS-self-reactive developing B cells are eliminated specifically from the meninges via the in situ tolerance checkpoint.
“There has been a knowledge gap on how exactly B cell tolerance selection against highly tissue-specific antigens is achieved if the event only occurs in the bone marrow” said Heping Xu PhD, an immunologist and Assistant Professor in the School of Life Sciences, Westlake University. Xu is the co-senior author on the paper. “We noticed that B cells are present in the meninges according to recent publications, but whether and how potentially self-reactive B cells respond to CNS-specific antigens in the meninges is unknown. What we’ve found is that there’s a new and conserved in situ negative selection mechanism to ensure a locally non-self-reactive immune repertoire.”
“Understanding how immune repertoire is shaped at the brain borders under homeostasis and diseases is essential to dissect the basic mechanisms underlying neuro-immune interactions” said Danyang He PhD, an Assistant Professor of Neuroimmunology in the School of Life Sciences, Westlake University. He co-advised the project and is the co-senior author on the paper. “The discovery of meningeal B cell development call for a reassessment of the current understanding of B cell development and tolerance checkpoints, and shed light on the etiology of autoimmune encephalitis.”
Funding for this project provided in part by the National Key R&D program of China (grants 2020YFA0804200 and 2019YFA0802900), National Natural Science Foundation of China (grants U20A20346, 31970842 and 32070953), Westlake Laboratory of Life Sciences and Biomedicine and the Education Foundation of Westlake University.
Links: https://doi.org/10.1016/j.immuni.2021.09.016


