Dr. Bing Sun’s group found that the mechanism that allergens regulate IL-33 release from lung epithelial cells
Source:Bing Sun
2022-07-13
Dr. Bing Sun’s group from the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences published a research article in Nature Immunology on July 6th, 2022, entitled “Allergen protease-activated stress granule assembly and gasdermin D fragmentation control interleukin-33 secretion”. This study found the two cellular events regulated IL-33 secretion sequentially in the context of allergen protease exposure. First, the activation of SG assembly in airway epithelial cells licensed the nucleocytoplasmic transport of IL-33 but did not control its secretion. Second, the cleavage of Gsdmd at aa 309–313 (ELRQQ) generated p40 NT-Gsdmd, which promoted the cytosolic IL-33 to cross the membrane into the extracellular space.
Allergic diseases such as asthma and hay fever (allergic rhinitis) are common diseases observed with increasing incidence, especially in developed countries. These diseases are usually exacerbated when patients are environmentally exposed to allergens, such as asthma exacerbation induced by HDMs, molds, bacteria, plant pollen, and animal dander. Certain enzymatically active components derived from allergens, including Der p and Der f from HDMs, Asp f from Aspergillus oryzae, or the protease from Bacillus species induce cytokines release and promote allergic-type 2 airway inflammation in vivo.
IL-33 belongs to the IL-1 cytokine family and functions as an alarmin that is released when barriers are disrupted. The most well-defined and important functions of IL-33 rely on its ST2-binding ability, which activates Myd88-mediated signaling pathways in ST2-expressing cells such as mast cells, ILC2s, and the TH2 cells, contributing to systematic immune defenses and tissue repair. Thus, the secretion of IL-33 is critical for regulating allergic inflammation. Similar to other members of the IL-1 family, IL-33 lacks a secretion signal and cannot be released through the endoplasmic reticulum (ER)-Golgi secretory pathway or cross the cell membrane under physiological conditions. IL-33 was reported to be released through cell death-associated membrane damage, including those induced by freeze-thaw cycles, cell lysis buffer, pore-forming toxin streptolysin O (SLO), or oxidative stress. These stimuli contribute to IL-33 release with the occurrence of cell death features, such as LDH release or propidium iodide (PI) uptake. The secretion of IL-33 from living cells was also observed in cells exposed to mechanical stress, cockroaches, uric acid or HDMs, and Alternaria allergens. However, the precise mechanisms of the IL-33 secretion from living cells under allergen stimulation remain unknown, and the in-cell transportation of IL-33 has not been deciphered.
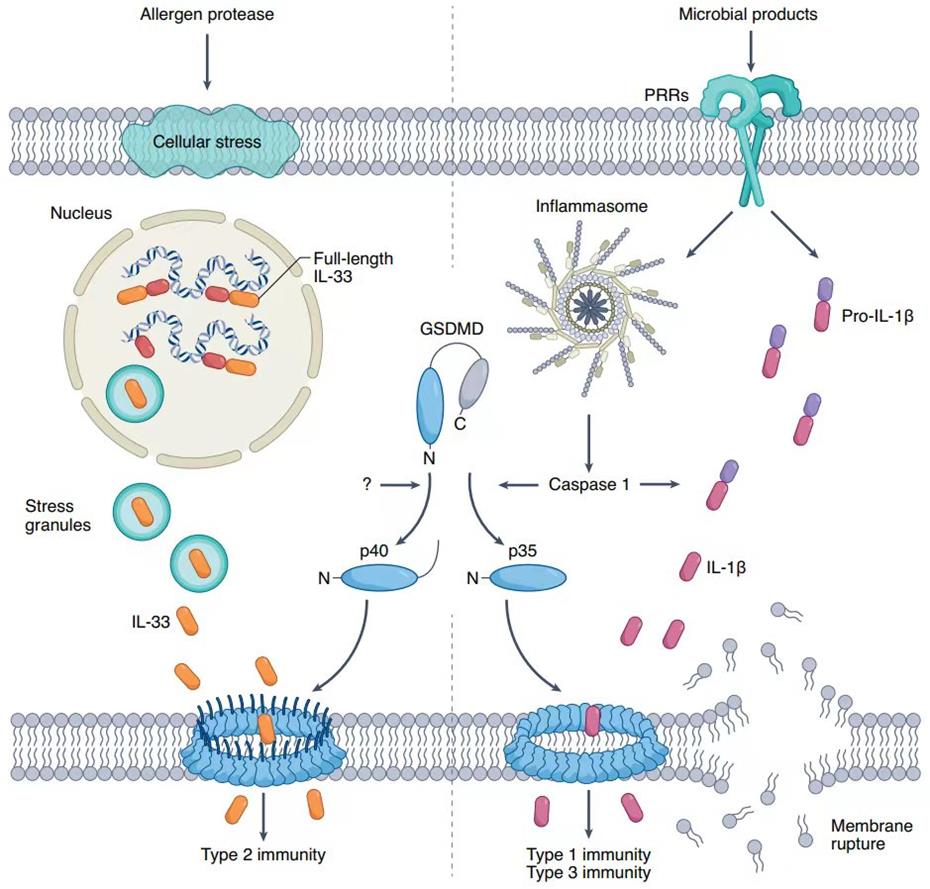
In this work, the authors found that two events sequentially regulated the secretion of IL-33 post-exposure to allergen proteases. The activation of p-eIF2α-independent SGs assembly in epithelial cells controlled the transport of IL-33 from the nucleus into the cytoplasm. Subsequently, Gsdmd, a gasdermin family member that controls the release of inflammatory IL-1β produced through the caspase-1/8/11-dependent pathway, was cleaved into a p40 N-terminal form (p40 NT-Gsdmd) through a caspase-independent mechanism. The generation of the p40 NT-Gsdmd fragment promoted the secretion of IL-33 from the cytosol into the extracellular space without the apparent occurrence of cell death. These observations provide two potential targets for interfering with IL-33-dependent inflammatory immune response following allergen proteases exposure.
Dr. Wen Chen, Dr. Shuangfeng Chen, Dr. Chenghua Yan, Dr. Yaguang Zhang, Dr. Ronghua Zhang from the Center for Excellence in Molecular Cell Science and Dr. Min Chen from Institute of Respiratory Diseases, Guangdong Medical College are co-first authors. Dr. Bing Sun, Dr. Yaguang Zhang from the Center for Excellence in Molecular Cell Science and Dr. Bin Wu, Dr. Dong Wu from Institute of Respiratory Diseases, Guangdong Medical College areco-corresponding authors. This work was supported by the Ministry of Science and Technology of China, the National Natural Science Foundation of China, Shanghai Science and Technology Innovation Action, the Youth Innovation Promotion Association of the Chinese Academy of Sciences, the Affiliated Hospital of Guangdong Medical University ‘Clinical Medicine+’CnTech Co-operation Project, the Discipline Construction Project of Guangdong Medical University, the Project of Zhanjiang City and the Natural Science Foundation of Guangdong Province. We thank F. Shao (National Institute of Biological Sciences, China) for helpful suggestions on the study and for providing Gsdmd-deficient mice and cell lines.
Links: https://www.nature.com/articles/s41590-022-01255-6
Allergic diseases such as asthma and hay fever (allergic rhinitis) are common diseases observed with increasing incidence, especially in developed countries. These diseases are usually exacerbated when patients are environmentally exposed to allergens, such as asthma exacerbation induced by HDMs, molds, bacteria, plant pollen, and animal dander. Certain enzymatically active components derived from allergens, including Der p and Der f from HDMs, Asp f from Aspergillus oryzae, or the protease from Bacillus species induce cytokines release and promote allergic-type 2 airway inflammation in vivo.
IL-33 belongs to the IL-1 cytokine family and functions as an alarmin that is released when barriers are disrupted. The most well-defined and important functions of IL-33 rely on its ST2-binding ability, which activates Myd88-mediated signaling pathways in ST2-expressing cells such as mast cells, ILC2s, and the TH2 cells, contributing to systematic immune defenses and tissue repair. Thus, the secretion of IL-33 is critical for regulating allergic inflammation. Similar to other members of the IL-1 family, IL-33 lacks a secretion signal and cannot be released through the endoplasmic reticulum (ER)-Golgi secretory pathway or cross the cell membrane under physiological conditions. IL-33 was reported to be released through cell death-associated membrane damage, including those induced by freeze-thaw cycles, cell lysis buffer, pore-forming toxin streptolysin O (SLO), or oxidative stress. These stimuli contribute to IL-33 release with the occurrence of cell death features, such as LDH release or propidium iodide (PI) uptake. The secretion of IL-33 from living cells was also observed in cells exposed to mechanical stress, cockroaches, uric acid or HDMs, and Alternaria allergens. However, the precise mechanisms of the IL-33 secretion from living cells under allergen stimulation remain unknown, and the in-cell transportation of IL-33 has not been deciphered.

In this work, the authors found that two events sequentially regulated the secretion of IL-33 post-exposure to allergen proteases. The activation of p-eIF2α-independent SGs assembly in epithelial cells controlled the transport of IL-33 from the nucleus into the cytoplasm. Subsequently, Gsdmd, a gasdermin family member that controls the release of inflammatory IL-1β produced through the caspase-1/8/11-dependent pathway, was cleaved into a p40 N-terminal form (p40 NT-Gsdmd) through a caspase-independent mechanism. The generation of the p40 NT-Gsdmd fragment promoted the secretion of IL-33 from the cytosol into the extracellular space without the apparent occurrence of cell death. These observations provide two potential targets for interfering with IL-33-dependent inflammatory immune response following allergen proteases exposure.
Dr. Wen Chen, Dr. Shuangfeng Chen, Dr. Chenghua Yan, Dr. Yaguang Zhang, Dr. Ronghua Zhang from the Center for Excellence in Molecular Cell Science and Dr. Min Chen from Institute of Respiratory Diseases, Guangdong Medical College are co-first authors. Dr. Bing Sun, Dr. Yaguang Zhang from the Center for Excellence in Molecular Cell Science and Dr. Bin Wu, Dr. Dong Wu from Institute of Respiratory Diseases, Guangdong Medical College areco-corresponding authors. This work was supported by the Ministry of Science and Technology of China, the National Natural Science Foundation of China, Shanghai Science and Technology Innovation Action, the Youth Innovation Promotion Association of the Chinese Academy of Sciences, the Affiliated Hospital of Guangdong Medical University ‘Clinical Medicine+’CnTech Co-operation Project, the Discipline Construction Project of Guangdong Medical University, the Project of Zhanjiang City and the Natural Science Foundation of Guangdong Province. We thank F. Shao (National Institute of Biological Sciences, China) for helpful suggestions on the study and for providing Gsdmd-deficient mice and cell lines.


