Prof. He Huang’s and Mingyao Liu’s team reported the breakthrough of non viral, specifically targeted CAR-T cells for relapsed/refractory non-hodgkin lymphoma
Source:He Huang
2022-10-11
Recently team of Professor Huang He/Hu Yongxian, chief physician, from the First Affiliated Hospital, School of Medicine, Zhejiang University, in collaboration with Professor Mingyao Liu and Jiqing Zhang’s team from Bangyao Biology/Huadong Normal University, reported a new strategy for CAR-T cell manufacture: using CRISPR/Cas9 gene editing technology to knock out PD1 in T lymphocytes accurately, insert CD19 CAR molecule targeted at tumor cells at a fixed point, and construct non-viral, specifically targeted CAR-T cells (PD1-19bbz), and completed a phase I clincial trial. Research results were published entitled "Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL " in Nature on August 31, 2022.
CRISPR/Cas9-mediated non-viral site-specific integration of CAR-T cell technology can effectively solve several problems caused by the use of viral vectors, showing great advantages. Site-specific integration can accurately insert each CAR sequence into a specific site in the genome, avoid the risk of tumorigenesis caused by random insertion, and ensure the safety and effectiveness of CAR-T products to the greatest extent. Only one step of preparation is needed to realize the continuous expression of CAR and the regulation of endogenous genes of T cells at the same time, which greatly shortens the preparation time of the whole CAR-T product and benefits more patients. In addition, the use of non-viral production technology can also greatly reduce the high cost caused by the use of viral vectors.
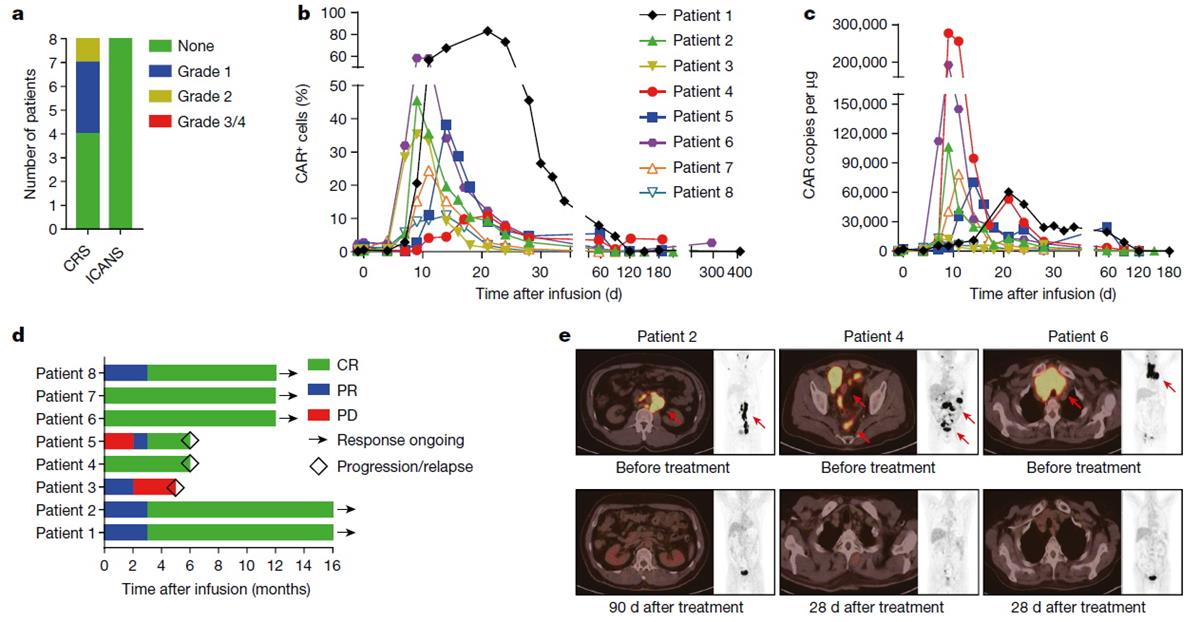
This study is the first time in the world to use CRISPR/Cas9 gene editing technology to achieve site-specific integration of CAR-T cells at PD1 site, and it is also the first clinical study in the world to treat lymphoma patients with non-viral, PD1 site-specific integration of CAR-T cells. The results showed that the most CAR integrated cells can be obtained by homologous mediated repair (HDR) mechanism by using linear double-stranded DNA with homologous arm length of 800bp as template. PD1-19bbz CAR-T cells showed stronger and more lasting killing effect in tumor cells with high or low expression of PD-L1, and the survival rate of mice was significantly improved. Further clinical research shows that no CAR-T-related neurotoxicity and cytokine release syndrome of grade 2 or above are observed in the treated patients, which proves that PD1-19bbz CAR-T cells have excellent clinical safety. PCR and flow cytometry showed that PD1-19bbz CAR-T cells could be rapidly expanded and maintained for a long time in patients. In patients with refractory and relapsed lymphoma, the objective remission rate is as high as 100% and the complete remission rate is 87.5%. Furthermore, single cell sequencing showed that there were a high proportion of memory T cells in PD1-19bbz CAR-T cell products, and PD1-19bbz CAR-T cells in vivo had stronger tumor killing effect, and long-lasting CAR-T cells had the characteristics of memory cells. The mechanism behind the superior clinical efficacy of non-viral PD1 site-specific integration of CAR-T cells was comprehensively revealed.
This achievement was highly praised by Professor Justin Eyquem of the University of California and Victoria Aranda, senior editor of Nature: "An improved approach has been developed for producing precisely designed immune cells called CAR T cells that recognize and kill cancer cells. CAR T cells generated in this way were safe and showed potential therapeutic effects in individuals with a relapsed or treatment-resistant form of the immune-cell cancer called B-cell non-Hodgkin’s lymphoma.This study reports the first clinical trial using CAR T cells in which PD-1 is disrupted by integrating a CARencoding gene into its sequence. The high complete-remission rate with low associated toxicities is an exciting outcome and suggests that the CAR T cells are potent and safe. The authors also show the feasibility of using non-viral, gene-specific targeting in T cellsfor clinical application. This method will pave the way to more gene-targeted CAR-T-cell therapies, providing an important step for the field."
The success of this work means the establishment of a new CAR-T cell technology platform, and it is also the best clinical result of high remission rate and low toxicity in the treatment of refractory and relapsed lymphoma with CAR-T cells worldwide. It indicates that Chinese scholars are in an international leading position in research and development and clinical transformation of CAR-T cells.
Professor He Huang (the First Affiliated Hospital, School of Medicine, Zhejiang University ), Professor Mingyao Liu (Bangyao Biology), Professor Bing Du (Bangyao Biology) and Professor Dali Li (Bangyao Biology) are the co-corresponding authors of the paper. Jiqin Zhang (Huadong Normal University), Yongxian Hu (the First Affiliated Hospital, School of Medicine, Zhejiang University) and Jiaxuan Yang (Huadong Normal University) are the co-first authors of the paper. This project was funded by the Chinese National Natural Science Funds and Key R&D Program of Zhejiang Province.
Link: https://www.nature.com/articles/s41586-022-05140-y
CRISPR/Cas9-mediated non-viral site-specific integration of CAR-T cell technology can effectively solve several problems caused by the use of viral vectors, showing great advantages. Site-specific integration can accurately insert each CAR sequence into a specific site in the genome, avoid the risk of tumorigenesis caused by random insertion, and ensure the safety and effectiveness of CAR-T products to the greatest extent. Only one step of preparation is needed to realize the continuous expression of CAR and the regulation of endogenous genes of T cells at the same time, which greatly shortens the preparation time of the whole CAR-T product and benefits more patients. In addition, the use of non-viral production technology can also greatly reduce the high cost caused by the use of viral vectors.

This study is the first time in the world to use CRISPR/Cas9 gene editing technology to achieve site-specific integration of CAR-T cells at PD1 site, and it is also the first clinical study in the world to treat lymphoma patients with non-viral, PD1 site-specific integration of CAR-T cells. The results showed that the most CAR integrated cells can be obtained by homologous mediated repair (HDR) mechanism by using linear double-stranded DNA with homologous arm length of 800bp as template. PD1-19bbz CAR-T cells showed stronger and more lasting killing effect in tumor cells with high or low expression of PD-L1, and the survival rate of mice was significantly improved. Further clinical research shows that no CAR-T-related neurotoxicity and cytokine release syndrome of grade 2 or above are observed in the treated patients, which proves that PD1-19bbz CAR-T cells have excellent clinical safety. PCR and flow cytometry showed that PD1-19bbz CAR-T cells could be rapidly expanded and maintained for a long time in patients. In patients with refractory and relapsed lymphoma, the objective remission rate is as high as 100% and the complete remission rate is 87.5%. Furthermore, single cell sequencing showed that there were a high proportion of memory T cells in PD1-19bbz CAR-T cell products, and PD1-19bbz CAR-T cells in vivo had stronger tumor killing effect, and long-lasting CAR-T cells had the characteristics of memory cells. The mechanism behind the superior clinical efficacy of non-viral PD1 site-specific integration of CAR-T cells was comprehensively revealed.
This achievement was highly praised by Professor Justin Eyquem of the University of California and Victoria Aranda, senior editor of Nature: "An improved approach has been developed for producing precisely designed immune cells called CAR T cells that recognize and kill cancer cells. CAR T cells generated in this way were safe and showed potential therapeutic effects in individuals with a relapsed or treatment-resistant form of the immune-cell cancer called B-cell non-Hodgkin’s lymphoma.This study reports the first clinical trial using CAR T cells in which PD-1 is disrupted by integrating a CARencoding gene into its sequence. The high complete-remission rate with low associated toxicities is an exciting outcome and suggests that the CAR T cells are potent and safe. The authors also show the feasibility of using non-viral, gene-specific targeting in T cellsfor clinical application. This method will pave the way to more gene-targeted CAR-T-cell therapies, providing an important step for the field."
The success of this work means the establishment of a new CAR-T cell technology platform, and it is also the best clinical result of high remission rate and low toxicity in the treatment of refractory and relapsed lymphoma with CAR-T cells worldwide. It indicates that Chinese scholars are in an international leading position in research and development and clinical transformation of CAR-T cells.
Professor He Huang (the First Affiliated Hospital, School of Medicine, Zhejiang University ), Professor Mingyao Liu (Bangyao Biology), Professor Bing Du (Bangyao Biology) and Professor Dali Li (Bangyao Biology) are the co-corresponding authors of the paper. Jiqin Zhang (Huadong Normal University), Yongxian Hu (the First Affiliated Hospital, School of Medicine, Zhejiang University) and Jiaxuan Yang (Huadong Normal University) are the co-first authors of the paper. This project was funded by the Chinese National Natural Science Funds and Key R&D Program of Zhejiang Province.
Link: https://www.nature.com/articles/s41586-022-05140-y


