The orphan receptor Nur77 binds cytoplasmic LPS to activate the non-canonical NLRP3 inflammasome
Source:Pengyan Xia
2023-05-06
On March 30, 2023, Dr. Pengyan Xia's research team from the School of Basic Medical Sciences of Peking University and Dr. Shuo Wang's research team from the Institute of Microbiology of the Chinese Academy of Sciences jointly published a research paper entitled "The orphan receptor Nur77 binds cytoplasmic LPS to activate the non-canonical NLRP3 inflammasome" in Immunity. This study conducted in-depth exploration of the molecular mechanisms underlying the activation pathway of non-classical NLRP3 inflammasomes. This study identified a novel lipopolysaccharide intracellular receptor Nur77 protein and validated its important role in non-classical inflammasome signaling pathways. It provided a deep explanation of the intermediate processes of bridging caspase-11 activation and NLRP3 activation, supplementing important mechanisms in this field, and is expected to provide new targets for the development of sepsis treatment.
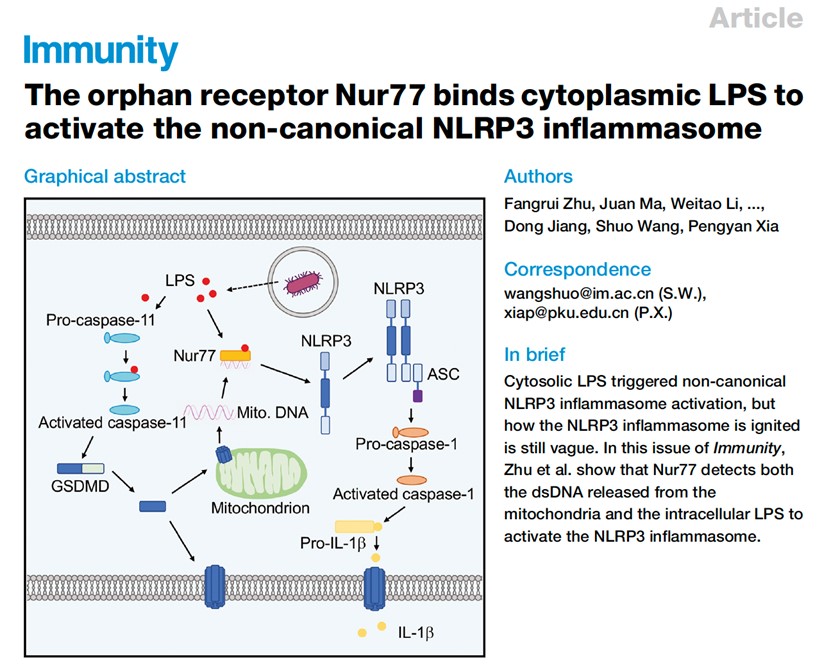
Caspase-11 can recognize intracellular lipopolysaccharide (LPS) and activate GSDMD, thereby activating NLRP3 inflammasomes, triggering caspase-1 cleavage and IL-1β release. This immune response is an important part of the host's response to pathogen infection. However, the specific mechanism by which caspase-11 triggers the activation of NLRP3 has always been an urgent challenge in this field. In this study, the intracellular binding proteins of LPS were identified by mass spectrometry, and the iBMDM knockout cell line of candidate proteins was constructed. After the stimulation of intracellular LPS, it was found that the secretion of IL-1β in Nr4a1 knockout cells was reduced but pyroptosis was not affected. Moreover, Nr4a1-/- BMDM cells showed normal activation of caspase-11 and GSDMD after being transferred to LPS, but no activation of caspase-1, indicating that Nur77 plays a role downstream of caspase-11 and upstream of NLRP3. In BMDM cells stimulated by intracellular LPS, the interaction between Nur77 and NLRP3 can be detected, and co-localization of Nur77 and NLRP3 within the cells can be observed through immunofluorescence staining. This indicates that Nur77 is activated through a regulatory pathway that interacts with NLRP3 during non-classical activation of inflammasomes. Researchers found that Nur77 can bind to NLRP3 only when LPS and dsDNA containing NBRE coexist. In the NLRP3 non-classical activation model, GSDMD punches holes in mitochondria to release mitochondrial DNA into the cytoplasm, which is important for activating Nur77. After being stimulated by intracellular LPS, cells lacking GSDMD no longer bind to NLRP3. Nur77 mutants, which lack LPS binding sites or DNA binding sites, cannot promote the activation of NLRP3. Researchers also tested the role of Nur77 in sepsis models, injecting LPS into wild-type and Nr4a1-/- mice pretreated with poly (I:C), and found a decrease in IL-1β in the serum of Nr4a1-/- mice. After isolating peritoneal macrophages from mice, it was found that Nr4a1 deficiency had no effect on pyroptosis. After injecting lethal doses of LPS, Nr4a1-/- mice survived longer, indicating that Nur77 promoted the host's response to endotoxin.
This work was conducted in the School of Basic Medical Sciences, Peking University. This research has been funded by the National Key Research and Development Program, the National Natural Science Foundation of China, the Chinese Academy of Sciences Strategic Leading Science and Technology Special Fund, the "Key Research Program of Frontier Science" of the Chinese Academy of Sciences, the Chinese Academy of Sciences Young Team in Stable Support of Basic Research, the Beijing Natural Science Foundation.
Article links:https://www.cell.com/immunity/fulltext/S1074-7613(23)00123-1

Caspase-11 can recognize intracellular lipopolysaccharide (LPS) and activate GSDMD, thereby activating NLRP3 inflammasomes, triggering caspase-1 cleavage and IL-1β release. This immune response is an important part of the host's response to pathogen infection. However, the specific mechanism by which caspase-11 triggers the activation of NLRP3 has always been an urgent challenge in this field. In this study, the intracellular binding proteins of LPS were identified by mass spectrometry, and the iBMDM knockout cell line of candidate proteins was constructed. After the stimulation of intracellular LPS, it was found that the secretion of IL-1β in Nr4a1 knockout cells was reduced but pyroptosis was not affected. Moreover, Nr4a1-/- BMDM cells showed normal activation of caspase-11 and GSDMD after being transferred to LPS, but no activation of caspase-1, indicating that Nur77 plays a role downstream of caspase-11 and upstream of NLRP3. In BMDM cells stimulated by intracellular LPS, the interaction between Nur77 and NLRP3 can be detected, and co-localization of Nur77 and NLRP3 within the cells can be observed through immunofluorescence staining. This indicates that Nur77 is activated through a regulatory pathway that interacts with NLRP3 during non-classical activation of inflammasomes. Researchers found that Nur77 can bind to NLRP3 only when LPS and dsDNA containing NBRE coexist. In the NLRP3 non-classical activation model, GSDMD punches holes in mitochondria to release mitochondrial DNA into the cytoplasm, which is important for activating Nur77. After being stimulated by intracellular LPS, cells lacking GSDMD no longer bind to NLRP3. Nur77 mutants, which lack LPS binding sites or DNA binding sites, cannot promote the activation of NLRP3. Researchers also tested the role of Nur77 in sepsis models, injecting LPS into wild-type and Nr4a1-/- mice pretreated with poly (I:C), and found a decrease in IL-1β in the serum of Nr4a1-/- mice. After isolating peritoneal macrophages from mice, it was found that Nr4a1 deficiency had no effect on pyroptosis. After injecting lethal doses of LPS, Nr4a1-/- mice survived longer, indicating that Nur77 promoted the host's response to endotoxin.
This work was conducted in the School of Basic Medical Sciences, Peking University. This research has been funded by the National Key Research and Development Program, the National Natural Science Foundation of China, the Chinese Academy of Sciences Strategic Leading Science and Technology Special Fund, the "Key Research Program of Frontier Science" of the Chinese Academy of Sciences, the Chinese Academy of Sciences Young Team in Stable Support of Basic Research, the Beijing Natural Science Foundation.
Article links:https://www.cell.com/immunity/fulltext/S1074-7613(23)00123-1


