Yifu Qiu’s group reveals that type 2 cytokines regulate aging
Source:Yifu Qiu
2024-03-04
On January 22, 2024, Dr. Yifu Qiu’s group from College of Future Technology and Center for Life Sciences at Peking University published a research article in Immunity, entitled “Type 2 cytokine signaling in macrophages protects from cellular senescence and organismal aging”. This study reveals that the IL-4-STAT6 pathway plays a key role in the senescence of macrophages through regulating transcription of genes related to DNA repair.
Cellular senescence and chronic inflammation are two hallmarks of aging, both of which are closely associated with immune system. In healthy individuals, immune system surveils and clears senescent cells. Thus, activation of immune system is a therapeutic option of age-associated diseases, and its aging would accelerate organismal aging. Senescence of immune cells (immunosenescence) is a significant advance in the aging field, serving as a promising target of age-related diseases such as cancer. Therefore, deciphering the mechanisms underlying immunosenescence would pave the way towards healthy aging, including immune cell types involved, induction, progression, and reversion of senescent immune cells.
To figure out the dynamics of immune system during aging, Zhao et al. found a dramatic decrease of key molecules (IL4R, IL13RA1 and STAT6) in type 2 immunity by analyzing human data from the GTEx database as well as mouse samples. Deletion of these genes (Il4-/-; Il13-/-, Stat6-/- or Il4ra-/-) accelerated aging in mice including reduced motor function and shorten lifespan. These findings suggest that the decrease of key molecules in type 2 immunity promotes aging.
As cellular senescence plays a central role in aging, the researchers set out to identify senescent cell types involved. Across multiple tissues, the expression of p16 and p21, two key markers of cellular senescence, was largely elevated in epididymal adipose tissue (eWAT) from Stat6-/- mice. Flow cytometry analysis showed that macrophages are the major contributor of p16 elevation. Macrophages from other tissues were also found to exhibit similar upregulation of p16 and p21. Furthermore, loss of STAT6 impairs the phagocytic function of bone marrow-derived macrophages (BMDMs). These results indicate that the deficiency of STAT6 induces macrophage senescence.
Macrophage-specific deletion of Stat6 (Stat6fl/fl;Lyz2Cre) decreased motor function and working memory in mice. On the molecular level, the expression of p16, p21 and senescence-associated secretory phenotype (SASP) was significantly upregulated in eWAT and liver. Thus, Stat6 deficiency-induced macrophage senescence accelerates aging in mice. Bone marrow transplantation and clodronate liposome-mediated macrophage clearance experiments further supported this point. Moreover, rapamycin treatment could partially ameliorate aging phenotypes of deficient mice via inhibition of SASP.
To understand the underlying mechanisms, they performed RNA-seq analysis and found that genes related to DNA repair were significantly downregulated in Stat6-/- macrophages. Meanwhile, Stat6-/- macrophages contained more cytosolic DNA from the nucleus. Previous studies demonstrate that the cGAS-STING pathway activated by free DNA can induce SASP and cellular senescence. Consequently, treatment with STING inhibitor H151 could rescue etoposide-induced senescence of Stat6-/- BMDMs. By a series of experiments including STAT6 point mutation, ChIP-seq, CUT&Tag-qPCR and dual luciferase reporter assay, they uncover the mechanism by which STAT6 can bind to the promoters of BRCA1 and UBE2T upon IL-4 stimulation, promoting their expression.
From a viewpoint of therapy, they investigated whether IL-4 administration could decelerate mouse aging. They found that IL-4 could promote DNA repair in macrophages, attenuating etoposide-induced senescence. Furthermore, production of IL-4 in liver by AAV-Il4 administration could improve the healthspan of aged mice.
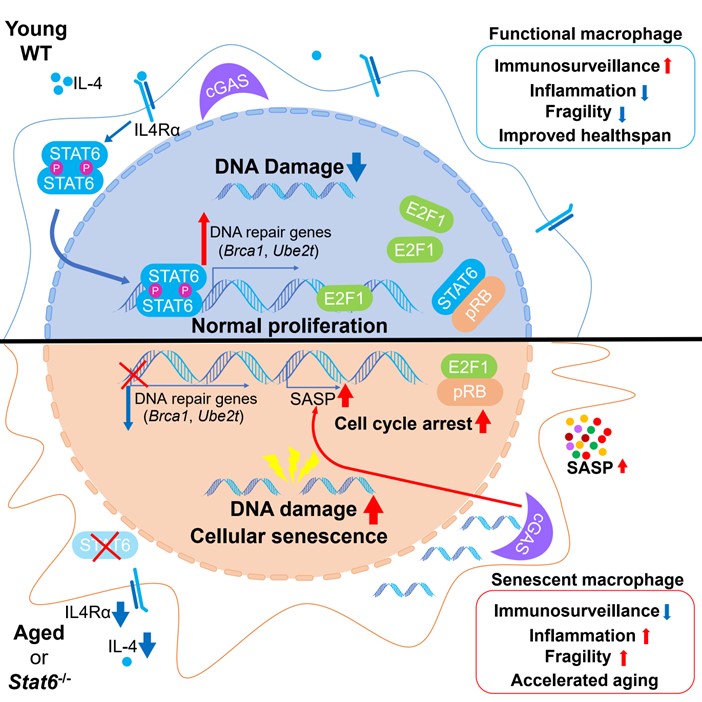
In summary, Qiu and colleagues discovered that the IL-4-STAT6 pathway controls DNA repair in macrophages. STAT6 promotes the expression of genes related to DNA repair upon IL-4 stimulation. In STAT6-deficient macrophages, impaired DNA repair leads to an accumulation of free DNA that activates cGAS-STING pathway and cellular senescence. On the other hand, senescent macrophages lose their immunosurveillance function, promoting chronic inflammation and organismal aging (Figure). Furthermore, proof-of-concept experiments in mice support a therapeutic potential of IL-4 in healthy aging.
The first author is Zhao Zhou, a fourth-year PhD student from College of Future Technology at Peking university. The corresponding author is prof. Yifu Qiu from College of Future Technology and Center for Life Sciences at Peking University. This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the PKU-Tsinghua Joint Center for Life Sciences.
Links: https://www.cell.com/immunity/fulltext/S1074-7613(24)00026-8
Cellular senescence and chronic inflammation are two hallmarks of aging, both of which are closely associated with immune system. In healthy individuals, immune system surveils and clears senescent cells. Thus, activation of immune system is a therapeutic option of age-associated diseases, and its aging would accelerate organismal aging. Senescence of immune cells (immunosenescence) is a significant advance in the aging field, serving as a promising target of age-related diseases such as cancer. Therefore, deciphering the mechanisms underlying immunosenescence would pave the way towards healthy aging, including immune cell types involved, induction, progression, and reversion of senescent immune cells.
To figure out the dynamics of immune system during aging, Zhao et al. found a dramatic decrease of key molecules (IL4R, IL13RA1 and STAT6) in type 2 immunity by analyzing human data from the GTEx database as well as mouse samples. Deletion of these genes (Il4-/-; Il13-/-, Stat6-/- or Il4ra-/-) accelerated aging in mice including reduced motor function and shorten lifespan. These findings suggest that the decrease of key molecules in type 2 immunity promotes aging.
As cellular senescence plays a central role in aging, the researchers set out to identify senescent cell types involved. Across multiple tissues, the expression of p16 and p21, two key markers of cellular senescence, was largely elevated in epididymal adipose tissue (eWAT) from Stat6-/- mice. Flow cytometry analysis showed that macrophages are the major contributor of p16 elevation. Macrophages from other tissues were also found to exhibit similar upregulation of p16 and p21. Furthermore, loss of STAT6 impairs the phagocytic function of bone marrow-derived macrophages (BMDMs). These results indicate that the deficiency of STAT6 induces macrophage senescence.
Macrophage-specific deletion of Stat6 (Stat6fl/fl;Lyz2Cre) decreased motor function and working memory in mice. On the molecular level, the expression of p16, p21 and senescence-associated secretory phenotype (SASP) was significantly upregulated in eWAT and liver. Thus, Stat6 deficiency-induced macrophage senescence accelerates aging in mice. Bone marrow transplantation and clodronate liposome-mediated macrophage clearance experiments further supported this point. Moreover, rapamycin treatment could partially ameliorate aging phenotypes of deficient mice via inhibition of SASP.
To understand the underlying mechanisms, they performed RNA-seq analysis and found that genes related to DNA repair were significantly downregulated in Stat6-/- macrophages. Meanwhile, Stat6-/- macrophages contained more cytosolic DNA from the nucleus. Previous studies demonstrate that the cGAS-STING pathway activated by free DNA can induce SASP and cellular senescence. Consequently, treatment with STING inhibitor H151 could rescue etoposide-induced senescence of Stat6-/- BMDMs. By a series of experiments including STAT6 point mutation, ChIP-seq, CUT&Tag-qPCR and dual luciferase reporter assay, they uncover the mechanism by which STAT6 can bind to the promoters of BRCA1 and UBE2T upon IL-4 stimulation, promoting their expression.
From a viewpoint of therapy, they investigated whether IL-4 administration could decelerate mouse aging. They found that IL-4 could promote DNA repair in macrophages, attenuating etoposide-induced senescence. Furthermore, production of IL-4 in liver by AAV-Il4 administration could improve the healthspan of aged mice.
In summary, Qiu and colleagues discovered that the IL-4-STAT6 pathway controls DNA repair in macrophages. STAT6 promotes the expression of genes related to DNA repair upon IL-4 stimulation. In STAT6-deficient macrophages, impaired DNA repair leads to an accumulation of free DNA that activates cGAS-STING pathway and cellular senescence. On the other hand, senescent macrophages lose their immunosurveillance function, promoting chronic inflammation and organismal aging (Figure). Furthermore, proof-of-concept experiments in mice support a therapeutic potential of IL-4 in healthy aging.

Figure. IL-4-STAT6 pathway decelerates aging via promoting DNA repair in macrophages
The first author is Zhao Zhou, a fourth-year PhD student from College of Future Technology at Peking university. The corresponding author is prof. Yifu Qiu from College of Future Technology and Center for Life Sciences at Peking University. This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the PKU-Tsinghua Joint Center for Life Sciences.
Links: https://www.cell.com/immunity/fulltext/S1074-7613(24)00026-8


