Tang Ce’s Team Redefines Innate Immunity in Pulmonary Fibrosis via Commensal Fungal Recognition
Source:Tang Ce
2025-07-22
On June 9, 2025, a collaborative study led by Dr. Ce Tang’s team at The First Affiliated Hospital of Sun Yat-sen University, together with researchers from Shenzhen Children's Hospital and Guangzhou University of Chinese Medicine, was published in Immunity. The study presents the first evidence that alveolar macrophages can be activated into a pro-fibrotic state through sensing commensal fungi translocated from the gut to the lung, establishing a new paradigm in the pathogenesis of fibrotic lung diseases. The findings offer novel mechanistic insights and therapeutic targets involving the interaction between innate immune cells and commensal fungi.
Background: A Missing Link Between Lung Microbiota and Fibrosis
Pulmonary fibrosis is a chronic and progressive interstitial lung disease characterized by alveolar destruction, fibroblast activation, and excessive deposition of extracellular matrix (ECM) components such as collagen, ultimately leading to respiratory failure. While classical pathways involving TGF-β signaling and matrix metalloproteinases (MMPs) have been extensively studied, the contribution of the lung-resident microbiota—especially fungi—remains largely unexplored.
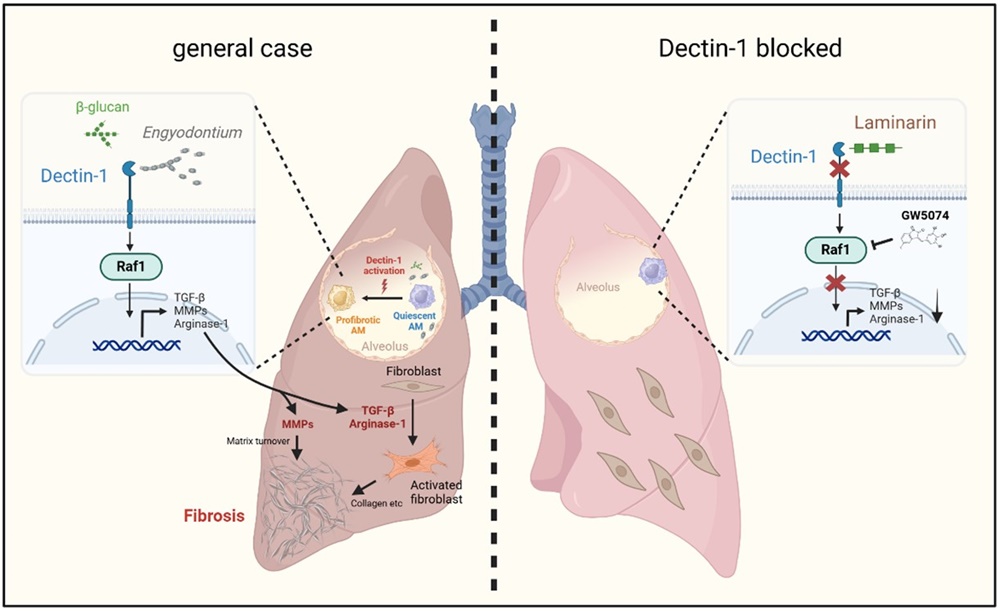
Alveolar macrophages are key sentinels of lung immunity, and their role in microbial sensing is well-established. Among the receptors expressed on innate immune cells, Dectin-1 (encoded by Clec7a) is a C-type lectin receptor that specifically recognizes β-glucans in fungal cell walls. While Dectin-1 has been widely implicated in antifungal defense, its role in fibrotic diseases was previously unclear.
Key Finding 1: Dectin-1 Expression Is Elevated in Fibrotic Lungs and Drives Macrophage Reprogramming
The researchers observed significantly increased expression of Dectin-1 in lung tissue samples from patients with idiopathic pulmonary fibrosis (IPF) and in a bleomycin-induced mouse model of lung fibrosis. Immunostaining and flow cytometry confirmed that this upregulation was largely restricted to alveolar macrophages.
In vitro and in vivo experiments demonstrated that Dectin-1 activation reprograms macrophages toward a pro-fibrotic phenotype, characterized by elevated expression of TGF-β and MMPs, thereby promoting ECM remodeling and fibroblast activation.
Key Finding 2: Engyodontium sp. Identified as a Lung-Colonizing Fungal Trigger of Fibrosis
To identify the upstream fungal ligand, the team utilized a Dectin-1 Fc fusion protein combined with ITS sequencing to isolate high-affinity fungal species from the lung microbiome. This led to the identification and culture of a Dectin-1-binding commensal fungus from the genus Engyodontium.
In murine models, lung colonization with Engyodontium sp. significantly exacerbated fibrotic pathology. Conversely, genetic deletion of Dectin-1 ameliorated fibrosis, confirming that this pathogenic effect is Dectin-1 dependent.
Key Finding 3: A Non-Canonical Dectin-1–Raf1 Axis Drives Fibrogenic Signaling
While CARD9 has long been considered a canonical adaptor for Dectin-1 signaling, neither CARD9 nor Dectin-2 knockout mice showed protection against fibrosis in this study. This led the researchers to investigate alternative downstream pathways.
They found that Raf1, a serine/threonine kinase, is a critical mediator of Dectin-1-induced pro-fibrotic signaling. Pharmacological inhibition of Raf1 significantly reduced fibrosis severity and collagen deposition, establishing the Dectin-1–Raf1 axis as a key pathway for fungal-induced fibrotic transformation in alveolar macrophages.
Scientific Significance: A Paradigm Shift in the Role of Alveolar Macrophages in Fibrosis
This study challenges the long-standing belief that pro-fibrotic macrophages originate solely from infiltrating monocytes. It demonstrates that resident alveolar macrophages can directly sense commensal fungi and adopt a fibrogenic phenotype, thereby reprogramming their function under disease conditions.
The discovery also emphasizes the importance of the gut–lung axis, wherein microbial translocation across mucosal barriers can impact distal immune responses and tissue remodeling. By targeting either the commensal fungus Engyodontium or the Dectin-1–Raf1 signaling cascade, new therapeutic strategies may be developed to treat pulmonary fibrosis and potentially other microbiota–immune interaction–driven chronic diseases.
Research Team and Acknowledgments
This study was co–first-authored by Ding Qiu (Master’s student), Dr. Shuishen Zhang (Associate Chief Thoracic Surgeon), and Dr. Chanyan Huang (Associate Chief Anesthesiologist), all from The First Affiliated Hospital of Sun Yat-sen University.
Dr. Ce Tang of the Precision Medicine Research Institute at The First Affiliated Hospital served as the lead corresponding author. Dr. Shaowei Dong (Shenzhen Children’s Hospital) and Dr. Shuai Wang (Guangzhou University of Chinese Medicine) are co–corresponding authors.
The research was supported by the National Natural Science Foundation of China and the Precision Medicine Research Institute at The First Affiliated Hospital. The study also received valuable contributions from international experts, including Professor Yoichiro Iwakura (University of Tokyo), Associate Professor Shigeru Kakuta, and Professor Tadashi Ando (Tokyo University of Pharmacy and Life Sciences).
Article link: https://doi.org/10.1016/j.immuni.2025.05.007
Background: A Missing Link Between Lung Microbiota and Fibrosis
Pulmonary fibrosis is a chronic and progressive interstitial lung disease characterized by alveolar destruction, fibroblast activation, and excessive deposition of extracellular matrix (ECM) components such as collagen, ultimately leading to respiratory failure. While classical pathways involving TGF-β signaling and matrix metalloproteinases (MMPs) have been extensively studied, the contribution of the lung-resident microbiota—especially fungi—remains largely unexplored.
Alveolar macrophages are key sentinels of lung immunity, and their role in microbial sensing is well-established. Among the receptors expressed on innate immune cells, Dectin-1 (encoded by Clec7a) is a C-type lectin receptor that specifically recognizes β-glucans in fungal cell walls. While Dectin-1 has been widely implicated in antifungal defense, its role in fibrotic diseases was previously unclear.
Key Finding 1: Dectin-1 Expression Is Elevated in Fibrotic Lungs and Drives Macrophage Reprogramming
The researchers observed significantly increased expression of Dectin-1 in lung tissue samples from patients with idiopathic pulmonary fibrosis (IPF) and in a bleomycin-induced mouse model of lung fibrosis. Immunostaining and flow cytometry confirmed that this upregulation was largely restricted to alveolar macrophages.
In vitro and in vivo experiments demonstrated that Dectin-1 activation reprograms macrophages toward a pro-fibrotic phenotype, characterized by elevated expression of TGF-β and MMPs, thereby promoting ECM remodeling and fibroblast activation.
Key Finding 2: Engyodontium sp. Identified as a Lung-Colonizing Fungal Trigger of Fibrosis
To identify the upstream fungal ligand, the team utilized a Dectin-1 Fc fusion protein combined with ITS sequencing to isolate high-affinity fungal species from the lung microbiome. This led to the identification and culture of a Dectin-1-binding commensal fungus from the genus Engyodontium.
In murine models, lung colonization with Engyodontium sp. significantly exacerbated fibrotic pathology. Conversely, genetic deletion of Dectin-1 ameliorated fibrosis, confirming that this pathogenic effect is Dectin-1 dependent.
Key Finding 3: A Non-Canonical Dectin-1–Raf1 Axis Drives Fibrogenic Signaling
While CARD9 has long been considered a canonical adaptor for Dectin-1 signaling, neither CARD9 nor Dectin-2 knockout mice showed protection against fibrosis in this study. This led the researchers to investigate alternative downstream pathways.
They found that Raf1, a serine/threonine kinase, is a critical mediator of Dectin-1-induced pro-fibrotic signaling. Pharmacological inhibition of Raf1 significantly reduced fibrosis severity and collagen deposition, establishing the Dectin-1–Raf1 axis as a key pathway for fungal-induced fibrotic transformation in alveolar macrophages.

Scientific Significance: A Paradigm Shift in the Role of Alveolar Macrophages in Fibrosis
This study challenges the long-standing belief that pro-fibrotic macrophages originate solely from infiltrating monocytes. It demonstrates that resident alveolar macrophages can directly sense commensal fungi and adopt a fibrogenic phenotype, thereby reprogramming their function under disease conditions.
The discovery also emphasizes the importance of the gut–lung axis, wherein microbial translocation across mucosal barriers can impact distal immune responses and tissue remodeling. By targeting either the commensal fungus Engyodontium or the Dectin-1–Raf1 signaling cascade, new therapeutic strategies may be developed to treat pulmonary fibrosis and potentially other microbiota–immune interaction–driven chronic diseases.
Research Team and Acknowledgments
This study was co–first-authored by Ding Qiu (Master’s student), Dr. Shuishen Zhang (Associate Chief Thoracic Surgeon), and Dr. Chanyan Huang (Associate Chief Anesthesiologist), all from The First Affiliated Hospital of Sun Yat-sen University.
Dr. Ce Tang of the Precision Medicine Research Institute at The First Affiliated Hospital served as the lead corresponding author. Dr. Shaowei Dong (Shenzhen Children’s Hospital) and Dr. Shuai Wang (Guangzhou University of Chinese Medicine) are co–corresponding authors.
The research was supported by the National Natural Science Foundation of China and the Precision Medicine Research Institute at The First Affiliated Hospital. The study also received valuable contributions from international experts, including Professor Yoichiro Iwakura (University of Tokyo), Associate Professor Shigeru Kakuta, and Professor Tadashi Ando (Tokyo University of Pharmacy and Life Sciences).
Article link: https://doi.org/10.1016/j.immuni.2025.05.007


