Hu Baoyang and Teng Zhaoqian's Team Reveals Key Mechanism by which TMEM119 Regulates Microglial Homeostasis in Alzheimer's Disease
Source:LIU Jing
2025-07-23
In July, 2025, a research team led by Dr. Baoyang Hu and Dr. Zhaoqian Teng at the Institute of Zoology, Chinese Academy of Sciences (CAS), and the Beijing Institute for Stem Cell and Regenerative Medicine, in collaboration with Dr. Yukai Wang and Prof. Junliang Yuan from Peking University Sixth Hospital, has published a research article titled "Microglial TMEM119 binds to amyloid-β to promote its clearance in an Aβ-depositing mouse model of Alzheimer's disease" in Immunity, a leading journal under Cell Press. This study is the first to systematically elucidate the functional role of the microglia-specific marker TMEM119 in the pathogenesis of Alzheimer’s disease (AD), uncovering its dual role in maintaining microglial homeostasis and promoting Aβ clearance. The findings identify TMEM119 as a novel therapeutic target and offer new strategies for AD intervention.
Alzheimer’s disease is a progressive neurodegenerative disorder characterized by β-amyloid (Aβ) deposition, neuronal loss, and abnormal microglial activation, posing a significant threat to cognitive health in the aging global population. Under normal conditions, microglia maintain homeostasis by expressing a set of signature markers, including TMEM119, CX3CR1, and P2RY12. However, during AD progression, microglia undergo substantial transcriptional reprogramming, transitioning from a homeostatic to a disease-associated microglia (DAM) state. While this transition is marked by the downregulation of homeostatic markers and upregulation of inflammatory genes, the functional role of TMEM119 in this process has remained unclear.
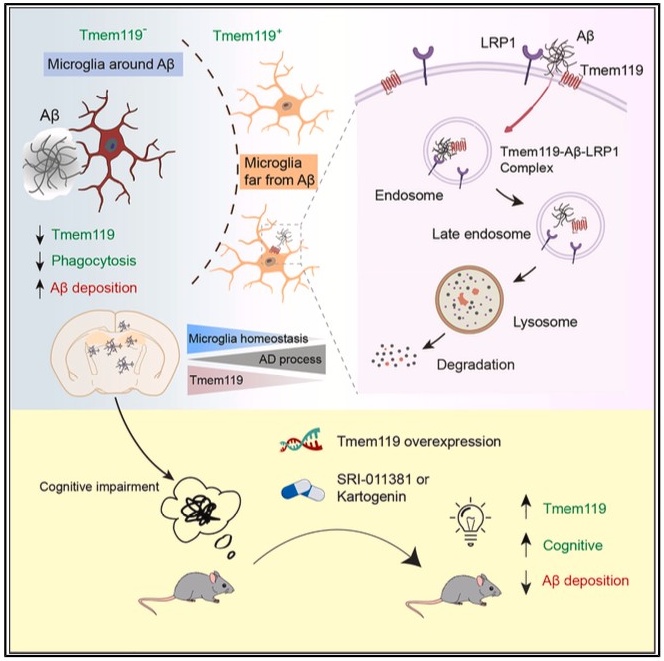
In this study, the researchers used the 5XFAD mouse model of AD and observed a marked reduction of TMEM119 expression around Aβ plaques, while expression remained intact in plaque-free regions. Further in vivo and in vitro analyses demonstrated that TMEM119 directly binds Aβ oligomers and recruits the Aβ-clearing receptor LRP1 to form a ternary complex that facilitates lysosomal degradation of Aβ. However, this process also results in proteolytic degradation of TMEM119 itself—revealing a “self-sacrificial” mechanism. These expression dynamics reflect microglial adaptation to pathological stress and explain the spatial heterogeneity of TMEM119 expression across different disease stages and brain regions.
Mechanistically, the study shows that loss of TMEM119 disrupts microglial homeostasis and accelerates their transition into DAM stage 1—a pro-inflammatory, early activation state. By contrast, another Aβ receptor, TREM2, primarily regulates the subsequent transition from DAM stage 1 to DAM stage 2, which is characterized by enhanced phagocytic activity. These findings highlight distinct yet complementary roles for TMEM119 and TREM2 in microglial fate determination and provide insights into the fine-tuned immune regulation in AD.
Moreover, the researchers identified two small-molecule compounds—KGN and SRI-011381—that enhance TMEM119 expression. Overexpression of TMEM119 or pharmacological upregulation via these compounds significantly improved Aβ phagocytosis in microglia and ameliorated cognitive deficits in mid-stage AD mouse models, supporting the translational potential of targeting TMEM119.
The team emphasizes that while mouse models offer experimental advantages, they differ from human AD in terms of disease progression speed and complexity. Therefore, further investigation into TMEM119 expression patterns and functions in the human brain is essential to bridge the gap between preclinical research and clinical application.
This landmark study not only fills a critical gap in understanding microglial state transitions in AD but also proposes a novel therapeutic concept centered on restoring microglial homeostasis. As a pivotal regulatory node in microglial biology, TMEM119 represents a promising entry point for developing precise interventions for Alzheimer’s disease.
This research was jointly conducted by the Institute of Zoology, Chinese Academy of Sciences; the Beijing Institute for Stem Cell and Regenerative Medicine; and Peking University Sixth Hospital. The study was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, and other funding agencies.
Article link:https://doi.org/10.1016/j.immuni.2025.04.018
Alzheimer’s disease is a progressive neurodegenerative disorder characterized by β-amyloid (Aβ) deposition, neuronal loss, and abnormal microglial activation, posing a significant threat to cognitive health in the aging global population. Under normal conditions, microglia maintain homeostasis by expressing a set of signature markers, including TMEM119, CX3CR1, and P2RY12. However, during AD progression, microglia undergo substantial transcriptional reprogramming, transitioning from a homeostatic to a disease-associated microglia (DAM) state. While this transition is marked by the downregulation of homeostatic markers and upregulation of inflammatory genes, the functional role of TMEM119 in this process has remained unclear.
In this study, the researchers used the 5XFAD mouse model of AD and observed a marked reduction of TMEM119 expression around Aβ plaques, while expression remained intact in plaque-free regions. Further in vivo and in vitro analyses demonstrated that TMEM119 directly binds Aβ oligomers and recruits the Aβ-clearing receptor LRP1 to form a ternary complex that facilitates lysosomal degradation of Aβ. However, this process also results in proteolytic degradation of TMEM119 itself—revealing a “self-sacrificial” mechanism. These expression dynamics reflect microglial adaptation to pathological stress and explain the spatial heterogeneity of TMEM119 expression across different disease stages and brain regions.
Mechanistically, the study shows that loss of TMEM119 disrupts microglial homeostasis and accelerates their transition into DAM stage 1—a pro-inflammatory, early activation state. By contrast, another Aβ receptor, TREM2, primarily regulates the subsequent transition from DAM stage 1 to DAM stage 2, which is characterized by enhanced phagocytic activity. These findings highlight distinct yet complementary roles for TMEM119 and TREM2 in microglial fate determination and provide insights into the fine-tuned immune regulation in AD.
Moreover, the researchers identified two small-molecule compounds—KGN and SRI-011381—that enhance TMEM119 expression. Overexpression of TMEM119 or pharmacological upregulation via these compounds significantly improved Aβ phagocytosis in microglia and ameliorated cognitive deficits in mid-stage AD mouse models, supporting the translational potential of targeting TMEM119.
The team emphasizes that while mouse models offer experimental advantages, they differ from human AD in terms of disease progression speed and complexity. Therefore, further investigation into TMEM119 expression patterns and functions in the human brain is essential to bridge the gap between preclinical research and clinical application.

This landmark study not only fills a critical gap in understanding microglial state transitions in AD but also proposes a novel therapeutic concept centered on restoring microglial homeostasis. As a pivotal regulatory node in microglial biology, TMEM119 represents a promising entry point for developing precise interventions for Alzheimer’s disease.
This research was jointly conducted by the Institute of Zoology, Chinese Academy of Sciences; the Beijing Institute for Stem Cell and Regenerative Medicine; and Peking University Sixth Hospital. The study was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, and other funding agencies.
Article link:https://doi.org/10.1016/j.immuni.2025.04.018


