Li Yulin Team Elucidates Novel Mechanism of Neuro-immune Regulation in Heart Failure via the Brain-Heart Axis
Source:Guoqui Li
2025-07-24
On July 8, 2025, a research team led by Professor Li Yulin from Beijing Anzhen Hospital, Capital Medical University, in collaboration with the Center for Cardiovascular Metabolism and Innovation at Guangdong Medical University, published a study titled "Optogenetic vagal nerve stimulation attenuates heart failure by limiting the generation of monocyte-derived inflammatory CCRL2⁺ macrophages" in the top-tier immunology journal Immunity.
Utilizing optogenetics, the researchers selectively activated choline acetyltransferase (ChAT)-expressing neurons within the dorsal vagal motor nucleus (DVMN). This approach precisely delineated the regulatory mechanism of the parasympathetic nervous system on cardiac immune cells, specifically CCRL2⁺ macrophages. The study revealed a novel neuro-immune axis wherein parasympathetic signaling suppresses cardiac inflammation and thereby protects cardiac function via the α7 nicotinic acetylcholine receptor (α7nAChR) pathway. These findings not only deepen our understanding of the pathogenesis of heart failure but also provide a theoretical foundation for developing more precise neuromodulation and immunotherapeutic strategies. A concurrent Preview article in Immunity, titled "Neuro-immune ChATing protects the heart", highlighted the critical role of neuro-immune interactions in heart failure.
Heart failure (HF) is a condition characterized by the heart's inability to meet circulatory demands due to left ventricular overload and remodeling, exhibiting high morbidity and mortality. Patients frequently display chronic activation of the sympathetic nervous system and diminished parasympathetic tone, particularly reduced vagal efferent activity. While the mechanisms underlying sympathetic hyperactivity and its clinical management (e.g., β-blockers) are well-studied, the specific roles and regulatory mechanisms of the parasympathetic nervous system in HF remain unclear. This knowledge gap partly stems from the inability of traditional vagus nerve stimulation techniques to precisely target and selectively activate vagal efferent fibers, thereby limiting precise investigation of parasympathetic function. The immune system, particularly cardiac macrophages, plays a pivotal role in the initiation and progression of HF. Although sympathetic regulation of cardiac and splenic inflammation has been reported, the role of the parasympathetic nervous system in modulating local cardiac inflammation and influencing HF outcomes remains incompletely elucidated.
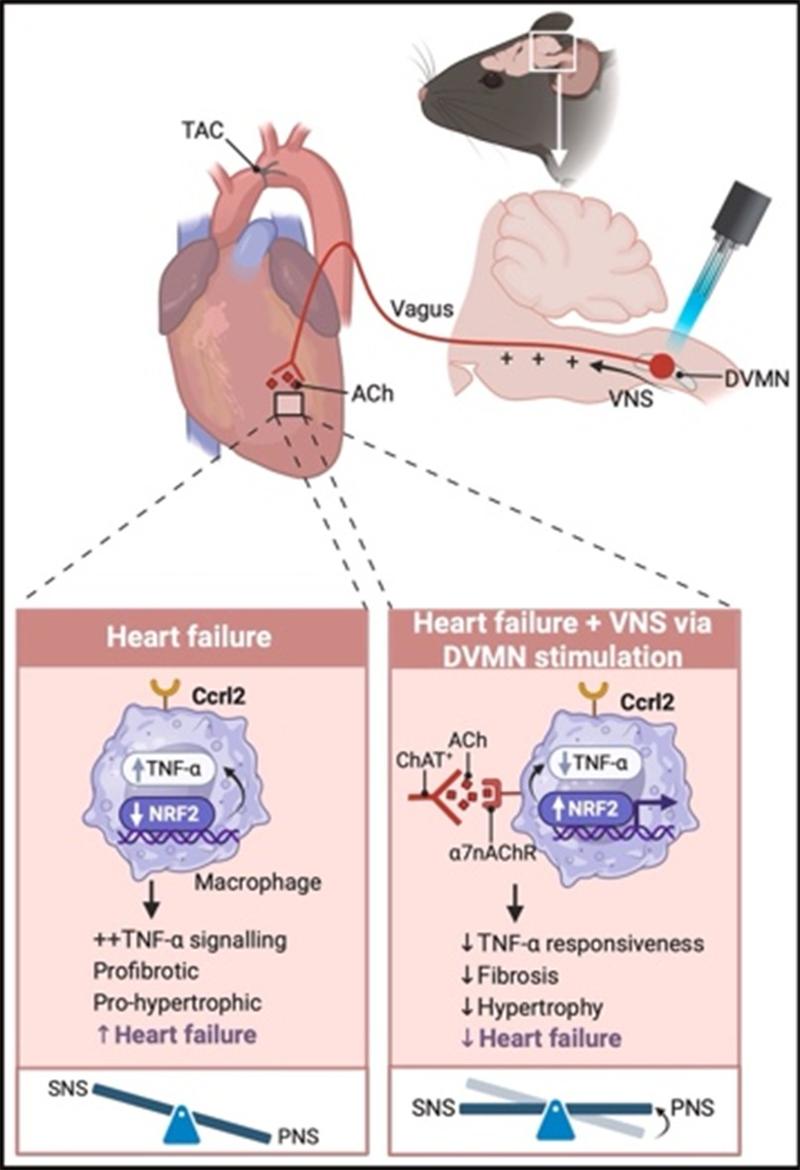
The research team employed optogenetic methods to precisely target and selectively activate ChAT⁺ neurons within the medullary DVMN. This effectively enhanced vagal nerve activity and attenuated heart failure progression. This cardioprotective effect is mediated through vagal nerve terminals acting on CCRL2⁺ non-classical macrophages in the heart: Activation of the α7nAChR receptor signaling pathway on these cells suppressed their pro-inflammatory, pro-fibrotic, and pro-apoptotic pathological functions, thereby inhibiting the development of heart failure. This work reveals for the first time the critical regulatory role of a parasympathetic-immune axis in heart failure.
Further investigations demonstrated that clinical HF patients and animal models exhibit significantly reduced vagal nerve activity, accompanied by decreased plasma acetylcholine levels and reduced numbers of ChAT⁺ neurons in the heart. Genomic association analysis confirmed a correlation between the CHRNA7 gene (encoding α7nAChR) and HF susceptibility. Furthermore, activating α7nAChR effectively suppressed the inflammatory response in CCRL2⁺ macrophages. Consequently, the authors propose several potential clinical intervention strategies, including targeted neuromodulation, pharmacological α7nAChR agonists, and lifestyle interventions (such as exercise and sleep regulation). These findings provide a new theoretical foundation and therapeutic rationale for future precise modulation of parasympathetic function to improve heart failure.
This study also highlights that the heart is not merely a pump but a complex system deeply regulated by neural and immune mechanisms. The parasympathetic nervous system plays a crucial role in the initiation and progression of HF through sophisticated "neuro-immune" crosstalk. HF management strategies targeting the nervous system are expanding beyond traditional sympathetic inhibition towards actively enhancing parasympathetic activity, potentially leading to novel therapeutic targets and clinical approaches in the future.
Guoqui Li (Assistant Researcher), Congcong Zhang (Researcher), and Professor Yang Li from the Institute of Cardiopulmonary Vascular Diseases, Beijing Anzhen Hospital, Capital Medical University, served as co-first authors. Professor Yulin Li (Beijing Anzhen Hospital, Capital Medical University), Professor Zhuofeng Lin (Center for Cardiovascular Metabolism and Innovation, Guangdong Medical University), and Professor Ping Li (Beijing Anzhen Hospital, Capital Medical University) are the corresponding authors.
Article link: https://www.cell.com/immunity/fulltext/S1074-7613(25)00272-9
Utilizing optogenetics, the researchers selectively activated choline acetyltransferase (ChAT)-expressing neurons within the dorsal vagal motor nucleus (DVMN). This approach precisely delineated the regulatory mechanism of the parasympathetic nervous system on cardiac immune cells, specifically CCRL2⁺ macrophages. The study revealed a novel neuro-immune axis wherein parasympathetic signaling suppresses cardiac inflammation and thereby protects cardiac function via the α7 nicotinic acetylcholine receptor (α7nAChR) pathway. These findings not only deepen our understanding of the pathogenesis of heart failure but also provide a theoretical foundation for developing more precise neuromodulation and immunotherapeutic strategies. A concurrent Preview article in Immunity, titled "Neuro-immune ChATing protects the heart", highlighted the critical role of neuro-immune interactions in heart failure.
Heart failure (HF) is a condition characterized by the heart's inability to meet circulatory demands due to left ventricular overload and remodeling, exhibiting high morbidity and mortality. Patients frequently display chronic activation of the sympathetic nervous system and diminished parasympathetic tone, particularly reduced vagal efferent activity. While the mechanisms underlying sympathetic hyperactivity and its clinical management (e.g., β-blockers) are well-studied, the specific roles and regulatory mechanisms of the parasympathetic nervous system in HF remain unclear. This knowledge gap partly stems from the inability of traditional vagus nerve stimulation techniques to precisely target and selectively activate vagal efferent fibers, thereby limiting precise investigation of parasympathetic function. The immune system, particularly cardiac macrophages, plays a pivotal role in the initiation and progression of HF. Although sympathetic regulation of cardiac and splenic inflammation has been reported, the role of the parasympathetic nervous system in modulating local cardiac inflammation and influencing HF outcomes remains incompletely elucidated.

Figure. DVMN ChAT+ optogenetic stimulation protects against HF by suppressing proinflammatory TNF-alpha signaling in Ccrl2+ macrophages via the α7nAChR–NRF2 pathway
The research team employed optogenetic methods to precisely target and selectively activate ChAT⁺ neurons within the medullary DVMN. This effectively enhanced vagal nerve activity and attenuated heart failure progression. This cardioprotective effect is mediated through vagal nerve terminals acting on CCRL2⁺ non-classical macrophages in the heart: Activation of the α7nAChR receptor signaling pathway on these cells suppressed their pro-inflammatory, pro-fibrotic, and pro-apoptotic pathological functions, thereby inhibiting the development of heart failure. This work reveals for the first time the critical regulatory role of a parasympathetic-immune axis in heart failure.
Further investigations demonstrated that clinical HF patients and animal models exhibit significantly reduced vagal nerve activity, accompanied by decreased plasma acetylcholine levels and reduced numbers of ChAT⁺ neurons in the heart. Genomic association analysis confirmed a correlation between the CHRNA7 gene (encoding α7nAChR) and HF susceptibility. Furthermore, activating α7nAChR effectively suppressed the inflammatory response in CCRL2⁺ macrophages. Consequently, the authors propose several potential clinical intervention strategies, including targeted neuromodulation, pharmacological α7nAChR agonists, and lifestyle interventions (such as exercise and sleep regulation). These findings provide a new theoretical foundation and therapeutic rationale for future precise modulation of parasympathetic function to improve heart failure.
This study also highlights that the heart is not merely a pump but a complex system deeply regulated by neural and immune mechanisms. The parasympathetic nervous system plays a crucial role in the initiation and progression of HF through sophisticated "neuro-immune" crosstalk. HF management strategies targeting the nervous system are expanding beyond traditional sympathetic inhibition towards actively enhancing parasympathetic activity, potentially leading to novel therapeutic targets and clinical approaches in the future.
Guoqui Li (Assistant Researcher), Congcong Zhang (Researcher), and Professor Yang Li from the Institute of Cardiopulmonary Vascular Diseases, Beijing Anzhen Hospital, Capital Medical University, served as co-first authors. Professor Yulin Li (Beijing Anzhen Hospital, Capital Medical University), Professor Zhuofeng Lin (Center for Cardiovascular Metabolism and Innovation, Guangdong Medical University), and Professor Ping Li (Beijing Anzhen Hospital, Capital Medical University) are the corresponding authors.
Article link: https://www.cell.com/immunity/fulltext/S1074-7613(25)00272-9


