Zhang Lianjun Team's Immunity Paper Uncovers a Novel Mechanism Underlying How Succinate Boosts Long-Term Anti-Tumor Immunity in CD8 T Cells
Source:Zhang Lianjun
2025-10-17
On October 14, 2025, a research team led by Prof. Lianjun Zhang from Suzhou Institute of Systems Medicine, the Chinese Academy of Medical Sciences, in collaboration with teams headed by Prof. Guideng Li, Prof. Zhimin Gu, and Prof. Ping-Chih Ho from the Ludwig Cancer Research at the University of Lausanne, Switzerland, published a study entitled "Succinate preserves CD8+ T cell fitness to augment antitumor immunity" at Immunity. The study reveals that succinate, a key intermediate of the tricarboxylic acid (TCA) cycle, can significantly enhance the survival, stemness maintenance, and long-term antitumor capacity of CD8+ T cells by activating mitophagy and inducing epigenetic remodeling. This discovery provides a novel metabolic intervention target for improving the efficacy of cancer immunotherapy.
Tumor antigen–specific CD8⁺ T cells are central executors of antitumor immunity, yet their function in the tumor microenvironment (TME) is often constrained by terminal exhaustion. T cell exhaustion is heterogeneous, and progenitor exhausted T cells (Tpex) with memory/stem-like signature are crucial for sustaining long-term immune memory and responses to immune checkpoint blockade. However, maintaining T cell stemness remains a major unresolved challenge in tumor immunology. The activation, differentiation, and function of T cells are finely regulated by both immune and metabolic signals. Succinate accumulates abnormally in tumors with succinate dehydrogenase (SDH) mutations. Yet, its role in CD8⁺ T cell–mediated antitumor immunity remains unclear. Therefore, clarifying how succinate regulates CD8⁺ T cell function and differentiation is essential not only to resolve existing controversies, but also to advance our understanding of metabolic reprogramming in antitumor immunity and to inform novel metabolism-targeted immunotherapies.
Using SDHB-deficient tumor models to mimic a succinate-rich microenvironment, the researchers found that succinate accumulation in tumors enhanced tumor-reactive CD8+ T cell–mediated immune responses. Sustained succinate exposure promoted CD8+ T cell survival and facilitated the generation and maintenance of stem-like subpopulations. Mechanistically, succinate enhanced mitochondrial fitness through BNIP3-mediated mitophagy and also promoted stemness-associated gene expression via epigenetic modulation. Succinate-conditioned CD8+ T cells displayed superior long-term persistence and tumor control capacity. Moreover, succinate enrichment correlates with favorable clinical outcomes in certain melanoma and gastric cancer patients receiving immune checkpoint blockade therapy. These findings reveal how succinate preserves T cell stemness and highlight the therapeutic potential of succinate supplementation for enhancing T cell immunotherapy efficacy.
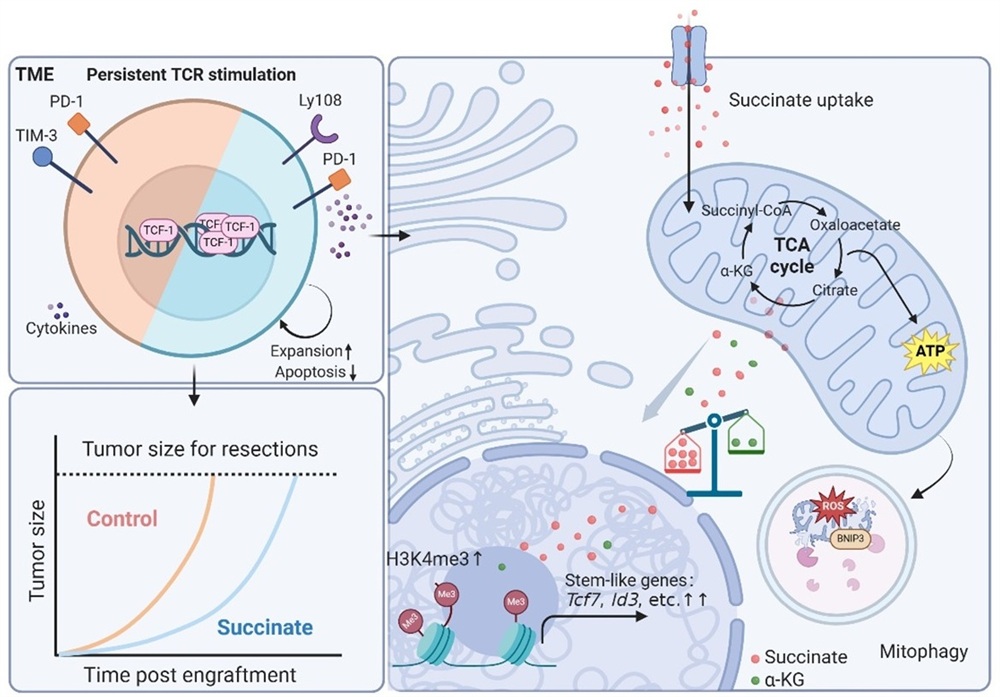
This study demonstrates that succinate enhances CD8⁺ T cell antitumor immunity through a dual pathway: metabolic adaptation and epigenetic remodeling. Succinate-induced stem-like T cells exhibit sustained persistence and retain differentiation capacity upon immune checkpoint blockade. This discovery deepens our understanding of metabolism–immunity crosstalk within the tumor microenvironment, and offers new theoretical and strategic insights for optimizing adoptive cell therapy and combination immunotherapy involving checkpoint inhibitors.
The research was in part supported by the National Natural Science Foundation of China, the Chinese Academy of Medical Sciences Innovation Fund, the National Center for Biotechnology Innovation's CGT Platform.
Article Link: https://www.sciencedirect.com/science/article/pii/S1074761325003267
Tumor antigen–specific CD8⁺ T cells are central executors of antitumor immunity, yet their function in the tumor microenvironment (TME) is often constrained by terminal exhaustion. T cell exhaustion is heterogeneous, and progenitor exhausted T cells (Tpex) with memory/stem-like signature are crucial for sustaining long-term immune memory and responses to immune checkpoint blockade. However, maintaining T cell stemness remains a major unresolved challenge in tumor immunology. The activation, differentiation, and function of T cells are finely regulated by both immune and metabolic signals. Succinate accumulates abnormally in tumors with succinate dehydrogenase (SDH) mutations. Yet, its role in CD8⁺ T cell–mediated antitumor immunity remains unclear. Therefore, clarifying how succinate regulates CD8⁺ T cell function and differentiation is essential not only to resolve existing controversies, but also to advance our understanding of metabolic reprogramming in antitumor immunity and to inform novel metabolism-targeted immunotherapies.
Using SDHB-deficient tumor models to mimic a succinate-rich microenvironment, the researchers found that succinate accumulation in tumors enhanced tumor-reactive CD8+ T cell–mediated immune responses. Sustained succinate exposure promoted CD8+ T cell survival and facilitated the generation and maintenance of stem-like subpopulations. Mechanistically, succinate enhanced mitochondrial fitness through BNIP3-mediated mitophagy and also promoted stemness-associated gene expression via epigenetic modulation. Succinate-conditioned CD8+ T cells displayed superior long-term persistence and tumor control capacity. Moreover, succinate enrichment correlates with favorable clinical outcomes in certain melanoma and gastric cancer patients receiving immune checkpoint blockade therapy. These findings reveal how succinate preserves T cell stemness and highlight the therapeutic potential of succinate supplementation for enhancing T cell immunotherapy efficacy.

Figure. Schematic illustration of succinate induced superior antitumor function of CD8⁺ T cells
This study demonstrates that succinate enhances CD8⁺ T cell antitumor immunity through a dual pathway: metabolic adaptation and epigenetic remodeling. Succinate-induced stem-like T cells exhibit sustained persistence and retain differentiation capacity upon immune checkpoint blockade. This discovery deepens our understanding of metabolism–immunity crosstalk within the tumor microenvironment, and offers new theoretical and strategic insights for optimizing adoptive cell therapy and combination immunotherapy involving checkpoint inhibitors.
The research was in part supported by the National Natural Science Foundation of China, the Chinese Academy of Medical Sciences Innovation Fund, the National Center for Biotechnology Innovation's CGT Platform.
Article Link: https://www.sciencedirect.com/science/article/pii/S1074761325003267


