Fu-Dong Shi Team’s Discovery Published in Science Reveals Novel Therapeutic Target for Neurological Diseases
Source:Zhiguo Li
2025-12-03
On November 14, 2025, Professor Fu-Dong Shi's team from Tianjin Medical University General Hospital/Beijing Tiantan Hospital has published a study in Science, titled "Targeting formyl peptide receptor 1 reduces brain inflammation and neurodegeneration." The research provides crucial insights into multiple sclerosis (MS) pathogenesis by demonstrating how formyl peptide receptor 1 (FPR1) drives regional brain inflammation and neurodegeneration – offering new hope for treating MS and related neurological disorders.
MS is a chronic inflammatory demyelinating disease of the central nervous system (CNS), which is the leading cause of non-traumatic disability in young adults worldwide. Its course involves localized immune inflammation and irreversible neurodegenerative damage in the brain. The existing MS treatment mainly target the peripheral immune system, and it is still difficult to effectively curb the continuous deterioration of brain lesions. This is manifested in: 1) Although the existing MS disease modifying drugs can effectively control the peripheral immune system and reduce MS recurrence, their therapeutic effect on chronic localized inflammation and degenerative lesions in the CNS is limited, and they cannot significantly delay brain atrophy or improve neurodegenerative diseases. Patients still face the risk of neurological disability progression. 2) MS neurodegeneration involves multiple mechanisms such as mitochondrial damage, oxidative stress, axonal demyelination, and chronic inflammation in brain regions, making it difficult to effectively intervene with a single target. Despite significant research resources invested globally over the past half century, no drug has been found that can effectively prevent MS neurodegeneration in clinical practice. Due to the prevalence of neuroinflammation and degeneration in almost all CNS diseases, billions of patients suffer from varying degrees of neurological dysfunction, resulting in a heavy burden on families, society, and the economy. Therefore, clarifying the molecular mechanisms of CNS inflammation and degeneration and developing corresponding treatment strategies has become a major focus of research in the fields of medicine and biology.
Mechanistic Insights into CNS Neurodegeneration
Building upon their June 2025 Science perspective article (PMID: 40536983), Academician Fu-Dong Shi and Academician Wee Yong state that CNS injuries, whether acute (traumatic brain injury, stroke, epilepsy, etc.) or chronic (multiple sclerosis, Alzheimer's disease, etc.), will trigger two core pathological processes, namely CNS inflammatory responses and neurodegeneration.
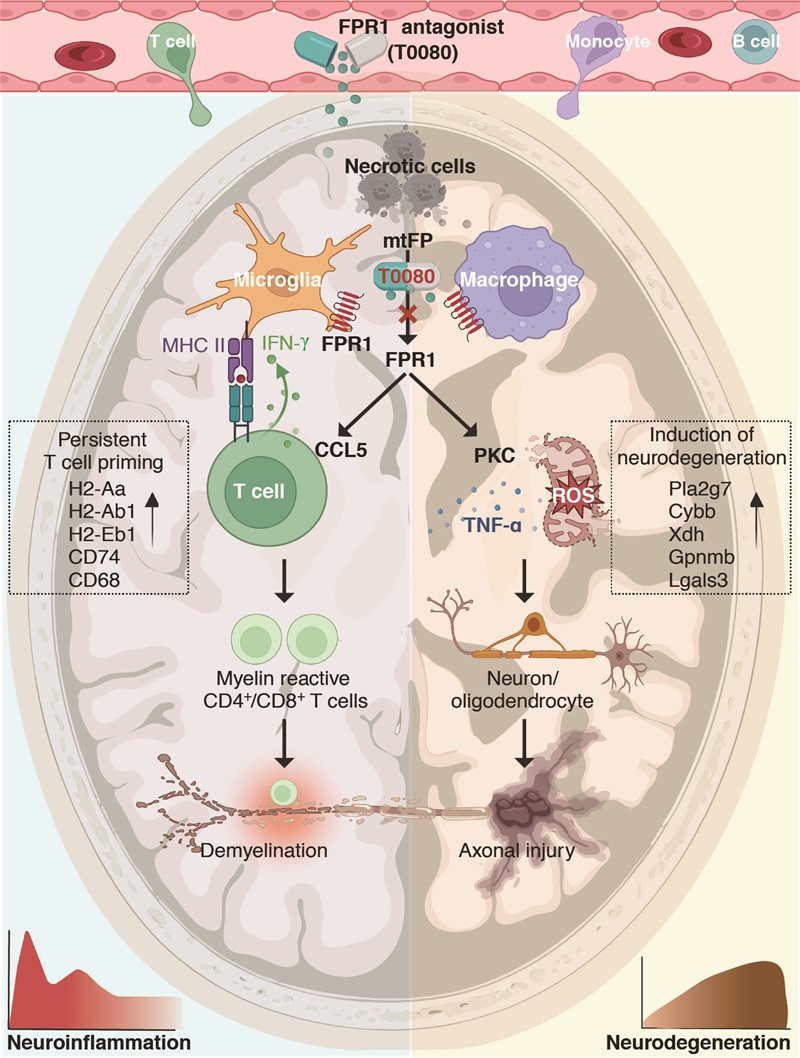
Regarding the neurodegenerative mechanisms in MS disease, Professor Shi's team discovered that damaged cells in MS lesions release mitochondrial formyl peptides, activating FPR1 on microglia and macrophages. Through single-cell sequencing, in situ pathology, and serum biomarker analysis, the researchers mapped the molecular network connecting cell necrosis, innate immune activation, and neurodegeneration in active MS lesions. Clinical data indicated the correlation between FPR1 and its ligand mitochondrial formyl peptide,lesion activity and disease progression in MS patients. In the mechanism study, it was found that on the one hand, FPR1 signaling enhances the antigen presentation ability of microglia, maintains the clonal expansion of CNS local myelin reactive T cells, and destroys myelin integrity; On the other hand, FPR1 signaling mediated neurodegeneration mainly regulates regional immune and inflammatory responses in the brain, rather than peripheral immune activation, revealing a novel neurodegenerative mechanism and potential therapeutic targets that differ from existing ones targeting peripheral immunity. In addition, FPR1 signaling enhances the release of neurotoxic factors such as reactive oxygen species from microglia and macrophages by activating protein kinase C, leading to typical neurodegenerative diseases such as neuronal apoptosis, axonal loss, mitochondrial dysfunction, and brain atrophy.
Screening and validating new therapeutic drugs
To intervene in FPR1 mediated inflammation and neurodegeneration in the CNS, the research team used computer-aided drug design and other techniques to screen and optimize the FPR1 small molecule antagonist T0080, which can cross the blood-brain barrier, from millions of small molecules. In all three MS mouse models, T0080 significantly inhibited FPR1 activation, reducing the progression of inflammation and neurodegeneration in the brain (Figure).
This study further improved the theory of CNS inflammation and degeneration mechanisms, and created a new FPR1 antagonist T0080 as a candidate drug for the treatment of MS. It may also provide new strategy for the treatment of diseases with common neurodegenerative features such as Alzheimer's disease, Parkinson's disease, and stroke.
Future Directions and Clinical Translation
Over the past two decades, it has been widely believed that the natural immune response mediated by peripheral migration of mononuclear macrophages and glial cells to the CNS is the main factor driving the progression of multiple sclerosis. Fu-Dong Shi’s team has been deeply involved in the field of neuroimmunology for a long time. Through his keen insight, he led the research team to trace the origin of myeloid cells. In 2022, he was published in Cell (Kaibin Shi et al., PMID: 35709748), revealing abnormal proliferation of myeloid cells in multiple sclerosis patients. Through the chemokine CCR5, these cells migrate to the CNS and promote disease progression. On this basis, they collaborated with Professor Hong Liu’s team from Shanghai Institute of Materia Medica to develop a new CCR5 inhibitor, Thioraviroc, which can effectively prevent myeloid cells from returning to the CNS, reduce neuroinflammation and degenerative diseases. It was approved for Class I clinical trials (IND) by the National Medical Products Administration in May 2025. At present, the Phase II clinical trial of Thioraviroc for the treatment of multiple sclerosis (THIRL-MS) has been initiated. The research results published in Science have once again achieved a breakthrough in the continuity of the progression mechanism of multiple sclerosis. At present, Fu-Dong Shi’s team is collaborating with the drug development company to actively optimize the characteristics of T0080 and promote its entry into clinical trials. In the future, it is expected to provide new treatment options for multiple sclerosis and other neurological diseases such as stroke and Alzheimer's disease.
The corresponding author of this study is Dr. Fu-Dong Shi, chief physician of the Department of Neurology at Tianjin Medical University General Hospital/Beijing Tiantan Hospital, and academician of the European Academy of Sciences; The co-first authors are Yulin Li, a resident physician and Zhiguo Li, an associate researcher at Tianjin Medical University General Hospital. The research group thanks Xuemei Han from China-Japan Union Hospital of Jilin University, Sheng Chen from Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Jinzhou Feng from the First Affiliated Hospital of Chongqing Medical University, Huaxing Meng from the First Affiliated Hospital of Shanxi Medical University, Jun Guo from Tangdu Hospital, Honghao Wang from Guangzhou First People's Hospital, Hongbo Liu from the First Affiliated Hospital of Zhengzhou University, and Friedemann Paul from Experimental and Clinical Research Center, Charité-Universitätsmedizin Berlin and Max Delbrueck Center for Molecular Medine in Germany for providing clinical data and samples of multiple sclerosis patients; And the collaborative works of many members of the Beijing Tianjin Center for Neuroimmunology (BTCN), including Wenyan He, Kaibin Shi, Minshu Li and Weina Jin. This project is supported by multiple funds including the National Natural Science Foundation of China.
Article Link: https://www.science.org/doi/10.1126/science.adq1177
MS is a chronic inflammatory demyelinating disease of the central nervous system (CNS), which is the leading cause of non-traumatic disability in young adults worldwide. Its course involves localized immune inflammation and irreversible neurodegenerative damage in the brain. The existing MS treatment mainly target the peripheral immune system, and it is still difficult to effectively curb the continuous deterioration of brain lesions. This is manifested in: 1) Although the existing MS disease modifying drugs can effectively control the peripheral immune system and reduce MS recurrence, their therapeutic effect on chronic localized inflammation and degenerative lesions in the CNS is limited, and they cannot significantly delay brain atrophy or improve neurodegenerative diseases. Patients still face the risk of neurological disability progression. 2) MS neurodegeneration involves multiple mechanisms such as mitochondrial damage, oxidative stress, axonal demyelination, and chronic inflammation in brain regions, making it difficult to effectively intervene with a single target. Despite significant research resources invested globally over the past half century, no drug has been found that can effectively prevent MS neurodegeneration in clinical practice. Due to the prevalence of neuroinflammation and degeneration in almost all CNS diseases, billions of patients suffer from varying degrees of neurological dysfunction, resulting in a heavy burden on families, society, and the economy. Therefore, clarifying the molecular mechanisms of CNS inflammation and degeneration and developing corresponding treatment strategies has become a major focus of research in the fields of medicine and biology.
Mechanistic Insights into CNS Neurodegeneration
Building upon their June 2025 Science perspective article (PMID: 40536983), Academician Fu-Dong Shi and Academician Wee Yong state that CNS injuries, whether acute (traumatic brain injury, stroke, epilepsy, etc.) or chronic (multiple sclerosis, Alzheimer's disease, etc.), will trigger two core pathological processes, namely CNS inflammatory responses and neurodegeneration.
Regarding the neurodegenerative mechanisms in MS disease, Professor Shi's team discovered that damaged cells in MS lesions release mitochondrial formyl peptides, activating FPR1 on microglia and macrophages. Through single-cell sequencing, in situ pathology, and serum biomarker analysis, the researchers mapped the molecular network connecting cell necrosis, innate immune activation, and neurodegeneration in active MS lesions. Clinical data indicated the correlation between FPR1 and its ligand mitochondrial formyl peptide,lesion activity and disease progression in MS patients. In the mechanism study, it was found that on the one hand, FPR1 signaling enhances the antigen presentation ability of microglia, maintains the clonal expansion of CNS local myelin reactive T cells, and destroys myelin integrity; On the other hand, FPR1 signaling mediated neurodegeneration mainly regulates regional immune and inflammatory responses in the brain, rather than peripheral immune activation, revealing a novel neurodegenerative mechanism and potential therapeutic targets that differ from existing ones targeting peripheral immunity. In addition, FPR1 signaling enhances the release of neurotoxic factors such as reactive oxygen species from microglia and macrophages by activating protein kinase C, leading to typical neurodegenerative diseases such as neuronal apoptosis, axonal loss, mitochondrial dysfunction, and brain atrophy.
Screening and validating new therapeutic drugs
To intervene in FPR1 mediated inflammation and neurodegeneration in the CNS, the research team used computer-aided drug design and other techniques to screen and optimize the FPR1 small molecule antagonist T0080, which can cross the blood-brain barrier, from millions of small molecules. In all three MS mouse models, T0080 significantly inhibited FPR1 activation, reducing the progression of inflammation and neurodegeneration in the brain (Figure).
This study further improved the theory of CNS inflammation and degeneration mechanisms, and created a new FPR1 antagonist T0080 as a candidate drug for the treatment of MS. It may also provide new strategy for the treatment of diseases with common neurodegenerative features such as Alzheimer's disease, Parkinson's disease, and stroke.

Figure. FPR1 and its antagonist T0080 in MS. The left hemisphere depicts an MS brain where FPR1 signaling sustains compartmentalized immune reactivity, compromising myelin integrity. The right hemisphere shows an atrophic brain of progressive MS as a result of FPR1-mediated neurodegeneration. The time course of neuroinflammation, neurodegeneration, and the site of action of FPR1 blockade by T0080 are also depicted. MHC II, major histocompatibility complex II.
Future Directions and Clinical Translation
Over the past two decades, it has been widely believed that the natural immune response mediated by peripheral migration of mononuclear macrophages and glial cells to the CNS is the main factor driving the progression of multiple sclerosis. Fu-Dong Shi’s team has been deeply involved in the field of neuroimmunology for a long time. Through his keen insight, he led the research team to trace the origin of myeloid cells. In 2022, he was published in Cell (Kaibin Shi et al., PMID: 35709748), revealing abnormal proliferation of myeloid cells in multiple sclerosis patients. Through the chemokine CCR5, these cells migrate to the CNS and promote disease progression. On this basis, they collaborated with Professor Hong Liu’s team from Shanghai Institute of Materia Medica to develop a new CCR5 inhibitor, Thioraviroc, which can effectively prevent myeloid cells from returning to the CNS, reduce neuroinflammation and degenerative diseases. It was approved for Class I clinical trials (IND) by the National Medical Products Administration in May 2025. At present, the Phase II clinical trial of Thioraviroc for the treatment of multiple sclerosis (THIRL-MS) has been initiated. The research results published in Science have once again achieved a breakthrough in the continuity of the progression mechanism of multiple sclerosis. At present, Fu-Dong Shi’s team is collaborating with the drug development company to actively optimize the characteristics of T0080 and promote its entry into clinical trials. In the future, it is expected to provide new treatment options for multiple sclerosis and other neurological diseases such as stroke and Alzheimer's disease.
The corresponding author of this study is Dr. Fu-Dong Shi, chief physician of the Department of Neurology at Tianjin Medical University General Hospital/Beijing Tiantan Hospital, and academician of the European Academy of Sciences; The co-first authors are Yulin Li, a resident physician and Zhiguo Li, an associate researcher at Tianjin Medical University General Hospital. The research group thanks Xuemei Han from China-Japan Union Hospital of Jilin University, Sheng Chen from Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Jinzhou Feng from the First Affiliated Hospital of Chongqing Medical University, Huaxing Meng from the First Affiliated Hospital of Shanxi Medical University, Jun Guo from Tangdu Hospital, Honghao Wang from Guangzhou First People's Hospital, Hongbo Liu from the First Affiliated Hospital of Zhengzhou University, and Friedemann Paul from Experimental and Clinical Research Center, Charité-Universitätsmedizin Berlin and Max Delbrueck Center for Molecular Medine in Germany for providing clinical data and samples of multiple sclerosis patients; And the collaborative works of many members of the Beijing Tianjin Center for Neuroimmunology (BTCN), including Wenyan He, Kaibin Shi, Minshu Li and Weina Jin. This project is supported by multiple funds including the National Natural Science Foundation of China.
Article Link: https://www.science.org/doi/10.1126/science.adq1177


